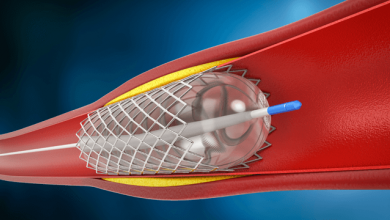
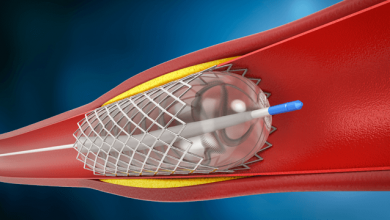
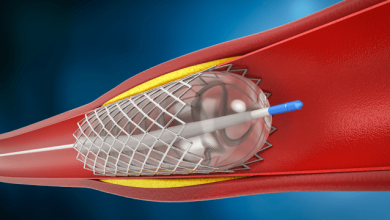
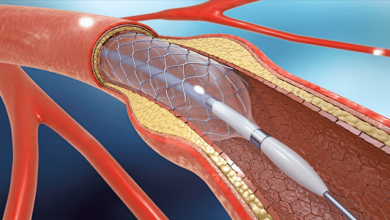
Drug-eluting balloons (DEB) are conventional semi-compliant angioplasty balloons covered with an anti-proliferative drug which is released into the vessel wall during inflation of the balloon, usually at nominal pressures with a specific minimal inflation time. The active substance on the DEB should be lipophilic enough to have a high absorption rate through tht vessel wall to compensate for the short period of contact between the inflated balloon and the vessel wall itself, and to maintain a sustained effect once released.
DEBs are typically used in re-stenotic lesions - previously stented segments that have developed neointimal hyperplasia and luminal loss. The advantage of this approach over the use of DES include a more homogeneous drug distribution and the fact that this mode of local delivery does not require foreign material implantation. Moreover, the recently introduced concept of “combined treatment strategy” of bare metal stent followed by DEB has aroused interest within interventional cardiology.
Although different DEBs appear similar in the underlying concept, the technical differences are important and include different coating techniques and applications of the different products in the market. As new information emerges over their potential indications and usage, there is a great deal to learn about DEBs.