Contents
Chapter 1: Introduction: The importance of preventing a secondary stroke
Chapter 2: Making the case for change
Chapter 3: Engaging the right stakeholders to set up the service
Chapter 4: Developing the right pathway
Chapter 5: Implanting the device
Chapter 6: Monitoring, follow-up and aftercare of patients post implant
1. Introduction: The importance of preventing a secondary stroke
This is David
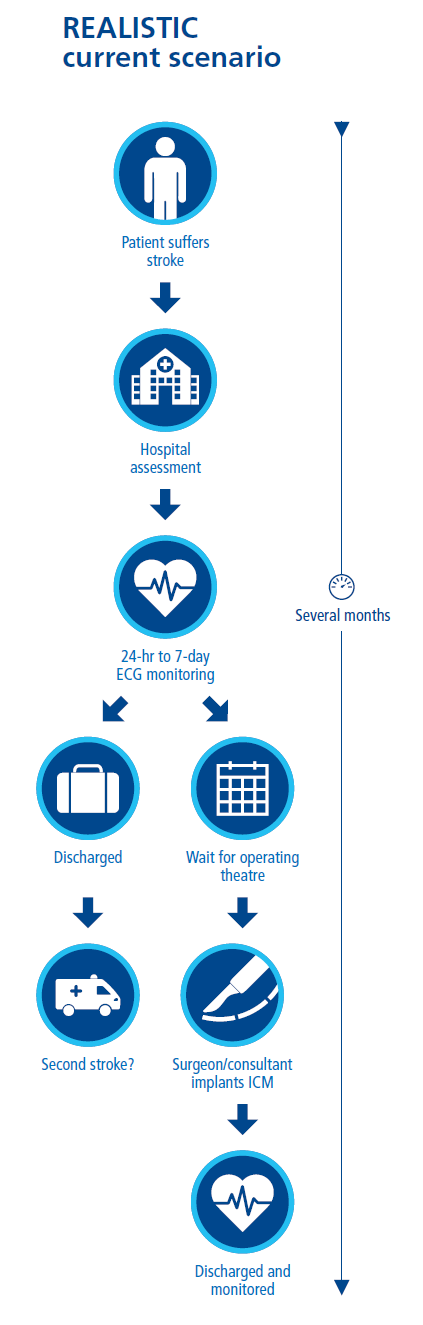
David is 55 years old and a senior manager at an IT company. He had a stroke last year, but the cause couldn’t be identified despite in-hospital investigations, including an electrocardiogram (ECG), 24-hour ambulatory monitor and echocardiogram. A diagnosis of cryptogenic stroke was made and he was discharged on antiplatelet therapy, blood pressure medication and statins. Of note, investigations did not identify the presence of paroxysmal atrial fibrillation (AF), so direct oral anticoagulants (DOACs) were not prescribed.
Two months ago, he had a devastating second stroke which left him with a dense hemiplegia and global dysphasia. His family were unable to give him the round-the-clock care he needed. Given his high dependence on care for activities of daily living, after 6 weeks in a stroke rehab unit, it was agreed David required ongoing care in a nursing home. His family is devastated and his dreams of the future are in tatters.
Whilst David’s story is fictitious, it does reflect a common and sadly preventable scenario. It is a moral imperative for us to ensure we identify the presence of paroxysmal AF where it has caused a stroke, so that we can prescribe the therapy we know is effective (Wilke et al., 2012) and reduce further cerebrovascular events. We have the technology to help us do this, and we have the evidence that it works. We now have support from NICE to do it (NICE Guideline DG41, 2020). We just need to get the pathway set up, work out the most efficient way to run the service – and then make it happen.
This report aims to help.
Undiagnosed AF is a pervasive problem on a population basis, with up to half a million people thought to be affected (Stroke Association, State of the Nation, 2018); people with AF have a risk of stroke that is around five times as high as those without AF (Wolf et al., 1991). Adequate treatment of AF could prevent around 7,000 strokes a year (Stroke Association, State of the Nation, 2018) – but it can only be treated if it is detected. Around 25% of embolic strokes are cryptogenic – in other words, they have no obvious cause (Saver, 2016). Patients who have had a cryptogenic stroke find themselves living in fear and at risk of it happening again (NICE Guideline DG41, 2020). Cryptogenic stroke patients as a secondary prevention group are a very vulnerable patient population, since parts of the brain already injured by the original stroke may not be as resilient.
The NHS Long-Term Plan lists early detection of AF as one of the priorities for care quality and outcomes improvement in the coming decade (NHS England, 2019). In light of the COVID-19 pandemic, it is even more important to implement the NICE DG41 Diagnostic Guidance as it enables monitoring stroke patients who might otherwise seek care after having a secondary stroke. In this context, detection of AF and secondary prevention of stroke presents a compelling opportunity and moral obligation for change, with positive outcomes likely to be seen quickly.
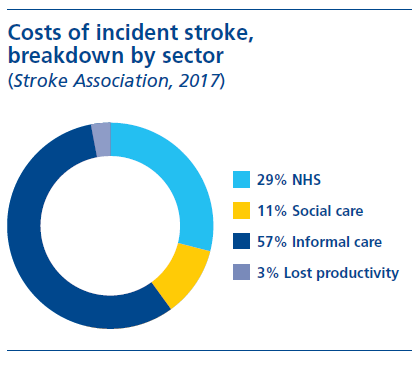
The consequences of a second AF-related stroke can be even more devastating. AF-related strokes are often more severe, with higher mortality and greater disability than strokes with other aetiologies: over a quarter of patients who are not anticoagulated before their AF stroke will die, with over 50% disabled (Stroke Association, AF: How can we do better?, 2018). In just the first 12 months after a stroke, the average societal cost of care (the impact on the NHS and social care as well as the costs of informal carers and productivity losses to society) is £45,409 – followed by £24,778 in subsequent years (Stroke Association, 2017). The overall costs attributed to stroke in the UK are approximately £25.6 billion per year (Stroke Association, 2017). The opportunity to prevent secondary stroke with better AF detection is therefore particularly striking: the number needed to treat (NNT) with DOACs to prevent a secondary stroke is just 17, compared with an NNT of around 25 for primary stroke (van Walraven et al., 2002; NHS England, 2019).
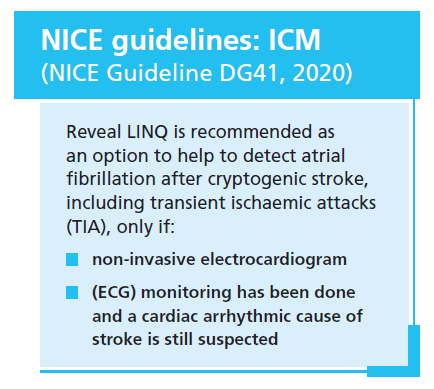
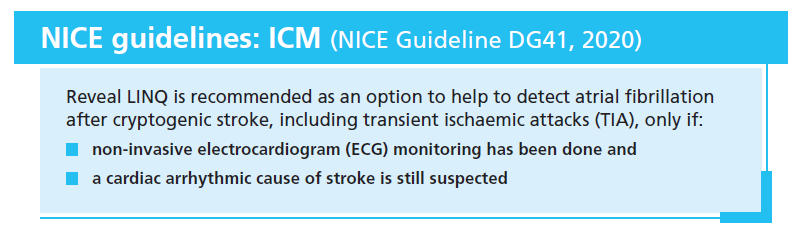
As recognised by the new NICE Diagnostic Guidance (NICE Guideline DG41, 2020), there are clinical trial data demonstrating that monitoring patients who have had a cryptogenic stroke (a subset of which is known as embolic stroke of undetermined source [ESUS]) with an implanted cardiac monitor (ICM) increases the detection of AF compared with shorter-term, external monitoring. ICMs can continuously monitor cardiac rhythm for 3–4 years after implantation, and are now recommended for patients with cryptogenic stroke where AF couldn’t be detected by external ECG monitoring and a cardiac arrhythmic cause of stroke is still suspected:
The pathway and process for implanting ICMs needs to be both efficient and effective, and consider already heavy workloads. At present, there is significant variation in access to ICMs as well as the efficiency of the pathway.
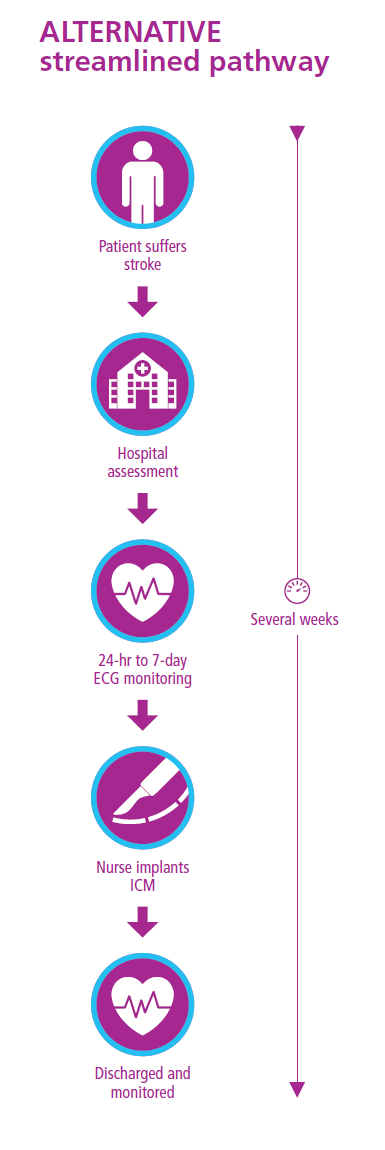
Among the authors of this report, we have pioneered the implementation of new, more streamlined pathways, utilising the abilities of nurses and electrophysiologists to free up other staff and to offer patients a more straightforward and smooth approach.
We know that setting up a new service is not easy and requires clinical energy and will, process mapping and service redesign, making and approving the business case, project management and clinical resource and monitoring processes. There are barriers to some of these which will be unique to each local system, and often boil down to funding, priority and clinical resource.
One of the keys to success is to learn from others who have already done it. Although every Trust has slightly different needs and resource levels, having been through the process and set up successful services, our experiences can help others to save time and workload when repeating the process in their own centre. This report contains those experiences, condensed into short summaries of steps that have worked for us and we feel could also work elsewhere.
With the new NICE Diagnostic Guidance on ICMs, now is the time to make this change. We hope that our experience will help you to set up a service that can prevent many more strokes and to protect patients like David from a second, devastating AF-related stroke.
References
-
BMJ Technology Assessment Group, 2019. Implantable cardiac monitors (BioMonitor 2-AF, Confirm Rx insertable cardiac monitor and Reveal LINQ Insertable Cardiac Monitoring System) to detect atrial fibrillation after cryptogenic stroke: Diagnostic assessment report. Available at: https://www.nice.org.uk/guidance/giddg10023/documents/diagnostics-assessment-report (last accessed October 2020).
-
NHS England, 2019. The NHS Long-Term Plan. Available at: https://www.longtermplan.nhs.uk/ (last accessed September 2020).
-
Saver JL, 2016. Clinical Practice: Cryptogenic Stroke. N Engl J Med 374(21):2065–2074.
-
Stroke Association, 2017. Current, future and avoidable costs of stroke in the UK. Available at: https://www.stroke.org.uk/sites/default/files/costs_of_stroke_in_the_uk_report_-executive_summary_part_2.pdf (last accessed October 2020).
-
Stroke Association, 2018. AF: How can we do better? Available at: https://www.stroke.org.uk/professionals/atrial-fibrillation-information-and-resources (last accessed October 2020).
-
Stroke Association, 2018. State of the nation: Stroke statistics. Available at: https://www.stroke.org.uk/resources/state-nation-stroke-statistics (last accessed October 2020).
-
van Walraven C, et al., 2002. Oral Anticoagulants vs Aspirin in Nonvalvular Atrial FibrillationAn Individual Patient Meta-analysis. JAMA 288(19):2441–2448.
-
Wilke T, et al., 2012. Oral anticoagulation use by patients with atrial fibrillation in Germany. Adherence to guidelines, causes of anticoagulation under-use and its clinical outcomes, based on claims-data of 183,448 patients. Thromb Haemost 107(6):1053–1065.
-
Wolf PA, et al., 1991. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22(8):983–988.
2. Making the case for change
The release of the new NICE guidance in 2020 (NICE Guideline DG41, 2020) recommending use of ICMs in selected patients with cryptogenic stroke provides a core rationale to anyone looking to make a business case for extended monitoring. However, there are a few important things to consider when building your case.
Making the case with numbers
Starting with the flaws in the current clinical pathway, and the development of a pathway is of excellence, is essential. This approach should be considered before you start to make the business case. A new pathway needs to promote the right clinical approach first and foremost (see chapter 3) after which the savings and indeed the business case follows.
Any business case needs to include both the activity and cost, but also the human angle. Below, we’ve provided some recommendations for important points to include – as well as some top tips from personal experience.
Many NHS budgets in the UK are currently system-controlled and during the COVID-19 pandemic, many acute hospitals are on a block contract: in the business case, we therefore need to make the very credible argument that reducing the chance of strokes in a population will translate to less burden on health and social care in the future. We know that AF affects an estimated 2–4% of the adult population, and the number of these patients is expected to at least double by 2050 (ESC Task Force, 2020). We also know that AF-related strokes are often more severe than non-AF-related strokes (Hahne et al., 2016; Saposnik et al., 2017), but effective anticoagulation therapy can decrease the rate of stroke by at least two thirds in AF (ESC Task Force, 2020) – this is of course only possible in cases where AF is identified. The costs of care following a stroke are significant: in the first 12 months after a stroke, the average total societal cost of care is £45,409 (Stroke Association, 2017). An additional £24,778 is incurred in subsequent years (Stroke Association, 2017). Detecting paroxysmal AF where it is present is therefore a critical goal to prevent secondary strokes and the financial, as well as human, consequences they bring – and one aligned with the priorities of the NHS long-term plan (NHS England, 2019).
We recommend including published data in the business case to demonstrate the sensitivity and specificity of ICMs. The randomized CRYSTAL-AF study showed that the Reveal XT (the predecessor to the Reveal-LINQ) implanted monitor detected over six times as many episodes of AF compared with standard follow-up after six months (Sanna et al., 2014) and almost ten times as many episodes by 36 months in patients with cryptogenic stroke (BMJ Technology Assessment Group, 2019). The NHS long-term plan notes that if 100 people with AF are identified and receive anticoagulation medication, an average of 4 strokes are averted, which translates to an NNT of 25 for primary prevention of stroke (NHS England, 2019).
It’s also important to mention the number needed to treat in order to prevent a secondary stroke: the NNT for secondary prevention with DOACs is just 17, compared with an NNT of around 25 for primary stroke (van Walraven et al., 2002; NHS England, 2019). In contrast, the NNT with statins in prevention of secondary MI, stroke or cardiovascular mortality in patients over 65 has been found to be around 60 (Lefeber et al., 2019). That’s why it is so essential, in addition to managing risk factors such as hypertension, smoking and high cholesterol, that we proactively identify patients who have paroxysmal AF and offer them anticoagulation to prevent a secondary stroke.
While there are additional costs associated with implementation of this approach, taking the big picture view should show that the long-term savings on a regional or population level will outweigh the costs. If we do not make this change, secondary strokes will increase as the population ages and grows. Avoidable pressures on care cost and resource will therefore escalate. Interventions to better detect and prevent secondary strokes would be expected to reduce costs associated with health and social care and treatment of secondary health conditions such as falls. The NICE Diagnostic Guidance (NICE Guideline DG41, 2020) has confirmed that ICMs are a cost-effective use of NHS resources. NICE considers NHS and social care costs in the assessment; however, informal care causes the majority of stroke costs, which are not considered by NICE (Stroke Association, 2017, NICE 2011). When adding these costs, ICMs are likely to generate long term cost savings.
Implementation of pathways using ICMs in patients with cryptogenic stroke has happened in Trusts across the UK, with great success. The case studies included in this document may help you to refer to the experiences of these pioneering centres in your case for change.
Making the case for moral responsibility
It is important to remember and to highlight in the case for change that implementation of an ICM pathway is both morally right and clinically appropriate: identifying the cause of cryptogenic stroke can help to avoid the devastating consequences of further strokes. In order to remind decision makers of this moral responsibility and to help to highlight the very human consequences of inaction, we recommend a business plan includes a patient voice – for example, the story of a real patient’s (or carer’s) experience and perspective on what this change would have meant for them.
Wider societal costs are harder to quantify than direct healthcare costs, but are nonetheless present following devastating strokes. There is the personal impact on the patient themselves: the patient’s quality of life is severely negatively impacted by a second stroke (Wang et al., 2014) and they can potentially no longer work. Since a quarter of strokes happen among people of working age (Stroke Association, 2017), the impact on employment can have broad societal costs in terms of productivity as well.
Long-term psychological impact, including depression, is common (Stroke Association, 2017; Stroke Association, 2020), with over 70% of stroke survivors experiencing depression or low mood (Stroke Association, A new era for stroke, 2018). The patient’s risk of additional health conditions (such as pneumonias and falls) is increased after a second stroke, reflected in the increased costs associated with care between 4 and 24 months after a recurrent stroke (Samsa et al., 1999).
And then there is the impact on friends and family. The patient might need round-the-clock care (over 50% are likely to be disabled [Stroke Association, AF: How can we do better? 2018]), affecting carers’ employment and social interactions. And the emotional impact on friends and family is enormous.
Additional points to raise and top tips
It’s essential to highlight that NICE guidance now recommends ICMs as an option to help to detect AF after a cryptogenic stroke (NICE Guideline DG41, 2020) as it means the robust cost-effective assessment of the technology has been completed. In addition, NICE’s Resource Impact Statement highlights the low impact on overall resource requirements in England (NICE Resource Impact Statement DG41, 2020).
It’s also important to highlight that the goal of reducing strokes is well aligned with the NHS longterm plan (NHS England, 2019).
Some systems might require a greater level of detail than others in the business case. Whatever level of detail you go for, it’s important to think about how the case should be nuanced when you present it – which will depend on the audience you’re speaking to.
References
-
BMJ Technology Assessment Group, 2019. Implantable cardiac monitors (BioMonitor 2-AF, Confirm Rx insertable cardiac monitor and Reveal LINQ Insertable Cardiac Monitoring System) to detect atrial fibrillation after cryptogenic stroke: Diagnostic assessment report. Available at: https://www.nice.org.uk/guidance/gid-dg10023/documents/diagnostics-assessment-report (last accessed October 2020).
-
ESC Task Force for the diagnosis and management of atrial fibrillation, 2020. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J doi:10.1093/eurheartj/ehaa612 (online ahead of print).
-
Go AS, et al., 2001. Prevalence of Diagnosed Atrial Fibrillation in Adults: National Implications for Rhythm Management and Stroke Prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) Study. JAMA 285:2370–2375.
-
Hahne K, et al., 2016. Atrial fibrillation and silent stroke: links, risks, and challenges. Vasc Health Risk Manag 12:65–74.
-
Lefeber GJ, et al., 2020. Statins After Myocardial Infarction in the Oldest: A Cohort Study in the Clinical Practice Research Datalink Database. J Am Geriatr Soc 68:329–336.
-
NHS England, 2019. The NHS Long-Term Plan. Available at: https://www.longtermplan.nhs.uk/ (last accessed September 2020).
-
NICE, 2011. Diagnostics Assessment Programme Manual. Available at: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-diagnostics-guidance (last accessed November 2020).
-
NICE, 2020. Implantable cardiac monitors to detect atrial fibrillation after cryptogenic stroke. Available at: https://www.nice.org.uk/guidance/dg41 (last accessed October 2020).
-
NICE, 2020. Resource impact statement. Available at: https://www.nice.org.uk/guidance/dg41/resources/resource-impact-statement-8836884829 (last accessed October 2020).
-
Samsa GP, et al., 1999. Epidemiology of Recurrent Cerebral Infarction: A Medicare Claims–Based Comparison of First and Recurrent Strokes on 2-Year Survival and Cost. Stroke 30:338–349.
-
Sanna T, et al., 2014. Cryptogenic Stroke and Underlying Atrial Fibrillation. N Engl J Med 370:2478–2486.
-
Saposnik G, et al., 2017. Atrial Fibrillation in Ischemic Stroke: Predicting Response to Thrombolysis and Clinical Outcomes. Stroke 44:99–104.
-
Stroke Association, 2017. Current, future and avoidable costs of stroke in the UK: Executive Summary, Part 2. Available at: https://www.stroke.org.uk/sites/default/files/costs_of_stroke_in_the_uk_report_-executive_summary_part_2.pdf (last accessed October 2020).
-
Stroke Association, 2018. AF: How can we do better? Available at: https://www.stroke.org.uk/professionals/atrial-fibrillation-information-and-resources (last accessed October 2020).
-
Stroke Association, 2018. A new era for stroke. Available at: https://www.stroke.org.uk/get-involved/campaigning/new-era-for-stroke (last accessed October 2020).
-
Stroke Association, 2020. Emotional changes after a stroke. Available at: https://www.stroke.org.uk/resources/emotional-changes-after-stroke (last accessed October 2020).
-
van Walraven C, et al., 2002. Oral Anticoagulants vs Aspirin in Nonvalvular Atrial FibrillationAn Individual Patient Meta-analysis. JAMA 288(19):2441–2448.
-
Wang Y-L, et al., 2014. Recurrent Stroke was Associated with Poor Quality of Life in Patients with Transient Ischemic Attack or Minor Stroke: Finding from the CHANCE Trial. CNS Neurosci Ther 20(12):1029–1035.
3. Engaging the right stakeholders to set up the service
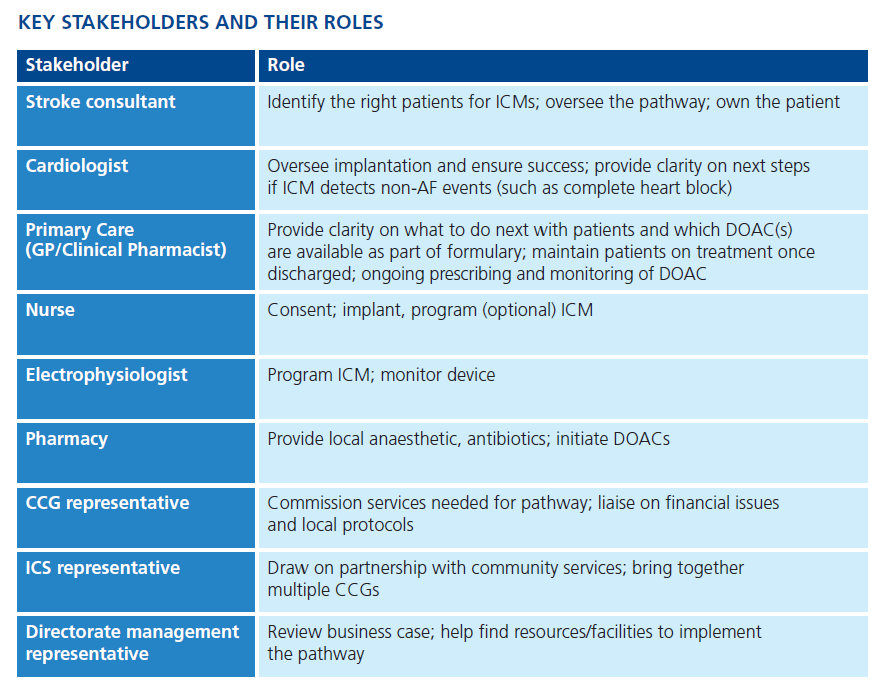
Even with the best possible pathway, to ensure change happens, we need close collaboration between key stakeholders and to have a champion (or champions) who will drive change forward. It’s not simply the case of developing a clear pathway and making a business case – it is about involving the right people – particularly from Stroke, Cardiology and Primary Care – to make change happen and having a strong, passionate champion to guide it through.
How should the different stakeholders work together?
While the overall clinical oversight and decision making should sit with the Stroke department, each stakeholder has an important role to play to develop the right pathway and once agreed, ensure the pathway is implemented and runs efficiently. We’d suggest holding a ‘kick-off’ meeting with representatives from each stakeholder at the start of the process to discuss all the points that need to be taken into account.
Each stakeholder should have clearly defined roles and responsibilities, so everyone owns part of the pathway. It’s important to make sure the group is not just hospital stakeholders – it needs to involve GP and CCG representation, and would ideally involve ICS level representation too. Involvement of all the relevant stakeholders is important to ensure local procedures and protocols are followed: for example, it’s important to ensure that if DOACs are prescribed to a patient, they will be available and recommended on the CCG formulary.
Learning from other Trusts is important to speed up the process, build confidence and potentially save time at kick-off. We’ve included case studies throughout this report to help you do this, and encourage you to reach out to the authors if you need additional support and advice.
Who should champion implementation?
A single overall champion in each Trust (or region) will help to drive the project forward and ensure smooth implementation. The pathway should be led by the Stroke department to ensure decision making sits with the stroke consultants; however, while the overall champion could be from this team, that doesn’t have to be the case. In at least one Trust, the overall champion was an electrophysiologist based in the cardiology department (see case study). The prerequisites for the overall champion of the new pathway are passion, drive and a relationship with all the key stakeholders – it’s crucial to keep everyone informed. Persistence will also be needed – with competing priorities, it’s important to make sure this doesn’t slip down people’s to-do lists too far!
As well as an overall champion, however, we also recommend a champion seated within each stakeholder: particularly within cardiology, general practice and the local CCG. These champions could attend the kick-off meeting recommended above.
The key starting point is to bring the right people together to develop the right pathway – after this, it is important to engage that group in creating a compelling business case. Having a group of stakeholders in place all focused on the same outcome will help make change happen, and will provide allies to help make sure the service does not hit any roadblocks.
Case Study:
Setting up an ICM pathway at Southend University Hospital
At Southend, the pathway setup was led and championed by the Principal Physiologist, who was also to be one of those responsible for implanting the device. The pathway moved the procedure out of the Catheter Laboratory into a designated room in the Coronary Care Unit, with implantation performed either by a cardiac physiologist or senior cardiology nurse.
The champion sourced devices and liaised with both the stroke department and with the CCG to keep them informed. A detailed protocol was initially developed by Stroke Consultants and distributed to the Cardiology team for comments and fine-tuning before joint agreement. This was then signed off by the Clinical Director, Cardiology and endorsed by the [TBC] Committee, and outlines clear roles and responsibilities for all staff involved in the procedure.
As one of the first Trusts to take this approach, obtaining initial sign-off was challenging – particularly with regard to prescribing local anaesthesia. However, evidence from this pioneering site can help others to make the same change, and we encourage you to reach out if you need support!
References
-
Royal College of Surgeons, 2017. Commissioning Guide: Carpal Tunnel Syndrome. Available at: https://www.rcseng.ac.uk/-/media/files/rcs/standards-and-research/commissioning/boa--carpal-tunnelsyndrome-guide-2017.pdf (last accessed October 2020).
-
Stroke Association, 2018. State of the nation: Stroke statistics. Available at: https://www.stroke.org.uk/resources/state-nation-stroke-statistics (last accessed October 2020).
4. Developing the right pathway
In order to set up and maintain a successful ICM monitoring service, the pathway must be clear. This requires a collaborative approach – but it’s been done before, and we hope our tips will help you to get it set up efficiently.
The pathway foundation: guidelines
The available NICE guidelines on ICM provide a solid basis for developing a pathway for investigation of patients with cryptogenic stroke or ESUS (NICE Guideline DG41, 2020). Ensuring specialists are broadly aware of these guidelines is important, particularly across both stroke and cardiology specialties.
Detection rate of new AF in cases of cryptogenic stroke using both noninvasive and invasive monitoring has been reported as between 5 and 17% (Kishore et al., 2014; Sposato et al., 2015; Kaura et al., 2019). Guidelines recommend (external) monitoring for 24 hours or longer where possible (RCP, 2016) to increase the chances of detection (RCP, 2016) – long-term monitoring has been shown to detect significantly more cases of AF compared with a 24-hour monitor (Gladstone et al,. 2014). It’s important this is carried out adequately before considering an ICM.
Designing the pathway
Care pathways should be locally driven in order to take local needs into account, but there are several key steps that should be present in each pathway. Between the local teams, a single standardised pathway should be developed, with a joint protocol agreed between all relevant stakeholders – especially between stroke and cardiology (see Chapter 2) to ensure the pathway is clear.
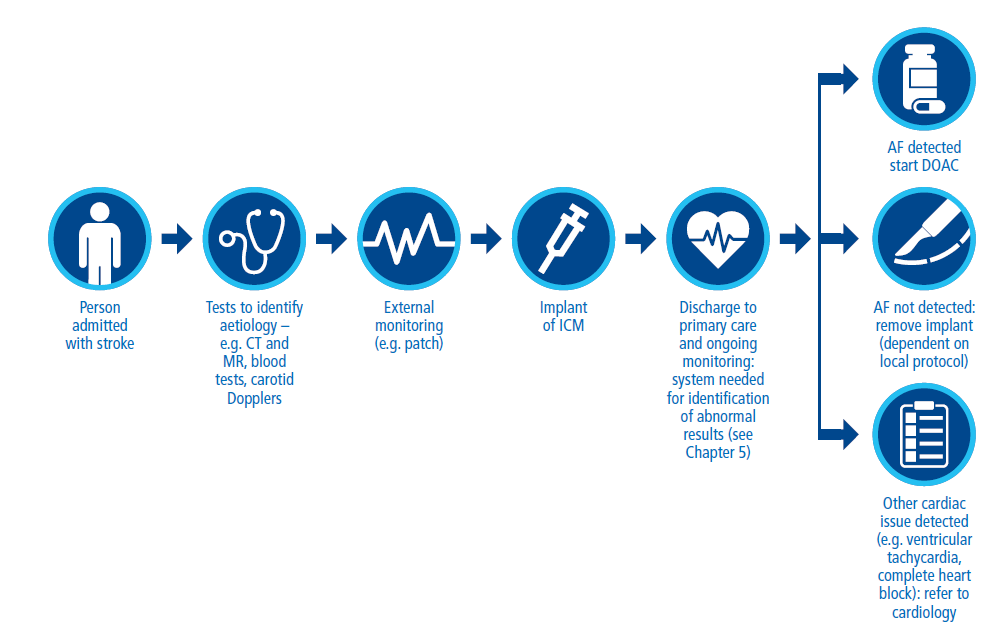
An initial key step is patient selection for ICM implantation. A thorough aetiological work-up should be performed, including intracranial and extracranial imaging (computed tomography [CT], magnetic resonance [MR] or Doppler), blood tests including lipid and Hba1c profiles, and ECG (with or without bubble contrast and/or a young stroke blood-screen (including haematological, biochemical, immunological and genetic screening) where relevant.
We recommend a robust arrhythmia detection pathway before inserting an ICM: non-invasive monitoring should be performed first, as recommended in the guidelines. Available options include 5- or 7-day ambulatory ECG, a multi-day event recorder or R-test or 2-week patch monitoring (Kishore et al., 2020; Thomas et al., 2018).
Multidisciplinary team collaboration is essential. Early multidisciplinary agreement on clear definitions (for example of vascular disease), patient needs and suitability will help to prevent problems later on. Delivering in-house multidisciplinary cardiac monitoring, according to a structured protocol, can boost early detection rates and help to overcome resource and logistical challenges (Kishore et al., 2020).
In particular, referral to ICM implantation should be overseen by senior experts to ensure the patient is suitable – clear criteria should be agreed across stroke and cardiology during the development of the pathway, with oversight from senior consultants where there is uncertainty, to make sure the pathway is robust. With regard to monitoring following implantation, there should be a system of governance in place for non-AF rhythm findings (see Chapter 5).
Timeliness of investigations is critical – external monitoring should be started as soon as possible and continued for as long as is feasible (Higgins et al., 2013; Kaura et al., 2019; Kishore et al., 2020). If an arrhythmia is not found but still suspected, the patient should be referred quickly onto ICM. Some delays are inevitable, of course, but careful planning can help to reduce them. For example, with a clearly agreed pathway, patients confirmed as embolic stroke by the stroke department shouldn’t need a second assessment by cardiology prior to booking into the extended loop recorder clinic – this saves some time and makes the process more efficient.
Once patients are discharged, they will return to the care of their GP: here, clear communication is vital. Guidance should be supplied to the GP on next steps for the patient and on who owns their care in case the monitor picks up a signal (see Communication and collaboration between secondary and primary care, Chapter 5) – and if DOACs are needed, who should initiate treatment. To ensure this is implemented effectively and with all parties in agreement, it is important to ensure primary care representation when designing the pathway (see Chapter 2).
Implementing the pathway
Implementation will vary dependent on local resource and requirements – below, we have included some examples of how this has been done before, as a potential source of inspiration.
Case Study:
The Salford Royal experience and pathway
Patients are initially assessed with a standard 12-lead ECG device to monitor heart rhythm, followed by 5-day non-invasive ambulatory monitoring as an inpatient. Patients are eligible for ICM implantation if:
- They have ESUS, are considered suitable for long-term anticoagulation and have a modified Rankin score (mRs) of ≤3
- A range of stroke investigations has been conducted and paroxysmal atrial fibrillation (PAF) is still considered a possibility
The device should be implanted within 12 weeks following an acute stroke, either by an advanced nurse practitioner or a cardiac physiologist. A 3-monthly virtual follow-up is arranged by the cardio-respiratory investigations department and arrhythmias are highlighted to the referring physician and/or cardiologist. Monitoring continues until detection of arrhythmia or rundown of the battery (anticipated to be approximately 3 years post implant).
Case Study:
The UCL experience and pathway
Originally the plan was to develop a regional pathway, but it proved easier to start with a single Trust and scale-up. This pathway is in the process of agreement. UCL has a cardiology referral service on site but ICM are typically implanted at St Bartholomew’s hospital, who are commissioned for the service.
During development, it was key to have early dialogue with the cardiology department to ensure their support for a stroke-led ICM service, as well as to engage department managers and local commissioners to understand the current contracting, as they have many worthwhile projects they are trying to fund. It was important to highlight that we were not asking for an increase in number of ICM procedures, but rather reviewing barriers in the pathway to ensure timely AF detection. It was also important to have a standard operating procedure for the functioning of the clinic, identify an appropriate clinical area and liaise with microbiology to ensure the protocols met their approval. We clarified with information governance that this did not need any further approval (as it was not a new procedure) and developed an information leaflet and consent form. Early on, we started arranging training for our team by arranging observed clinics with the cardiology service. This also helped ensure we knew how to set up the device and to use the software for registering patients.
As part of the referral pathway for ICM, in addition to standard aetiological work-up, patients will receive 7–14-day patch monitoring for AF detection. Patients who remain ESUS after complete bloods, vascular and brain imaging, ECG and initial non-invasive cardiac monitoring, and who have mRs <4, will be referred on to a fortnightly joint stroke consultant/nurse practitioner-led ICM clinic. Any arrhythmias highlighted will be emailed through to the TIA clinic inbox, which is reviewed by a stroke consultant daily. If AF is detected, patients can be seen the next day in the daily TIA service for anticoagulation counselling.
References
-
Gladstone DJ, et al., 2014. Atrial Fibrillation in Patients with Cryptogenic Stroke. N Engl J Med 370(26):2467–2477.
-
Higgins P, et al., 2013. Noninvasive Cardiac Event Monitoring to Detect Atrial Fibrillation After Ischemic Stroke: A Randomized, Controlled Trial. Stroke 44(9):2525–2531.
-
Kaura A, et al., 2019. Early prolonged ambulatory cardiac monitoring in stroke (EPACS): an open-label randomised controlled trial. Eur J Med Res 24:25.
-
Kishore A, et al., 2014. Detection of Atrial Fibrillation After Ischemic Stroke or Transient Ischemic Attack: A Systematic Review and Meta-Analysis. Stroke 45:520–526.
-
Kishore A, et al., 2020. Quality Improvement in Atrial Fibrillation detection after ischaemic stroke (QUIT-AF). Clin Med 20(5):480–485.
-
NICE, 2020. Implantable cardiac monitors to detect atrial fibrillation after cryptogenic stroke. Available at: https://www.nice.org.uk/guidance/dg41 (last accessed October 2020).
-
Royal College of Physicians: Intercollegiate Stroke Working Party, 2016. National clinical guideline for stroke. Available at: https://www.rcplondon.ac.uk/guidelines-policy/stroke-guidelines (last accessed October 2020).
-
Sposato L, et al., 2015. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 4(4):377–387.
-
Thomas P, et al., 2018. Are we doing enough to detect paroxysmal atrial fibrillation after an acute ischaemic stroke? Survey of ECG monitoring methods among stroke physicians. Clin Med 18(3):264–266.
5. Implanting the device
Setting up an efficient process for implanting insertable cardiac monitoring (ICM) devices doesn’t need to be onerous. We’ve done it, and we’re happy to share our experiences to help make the process swift and painless. In this chapter, we consider the where, who, how and when of device implantation, along with what else needs to be considered.
Where can the device be implanted?
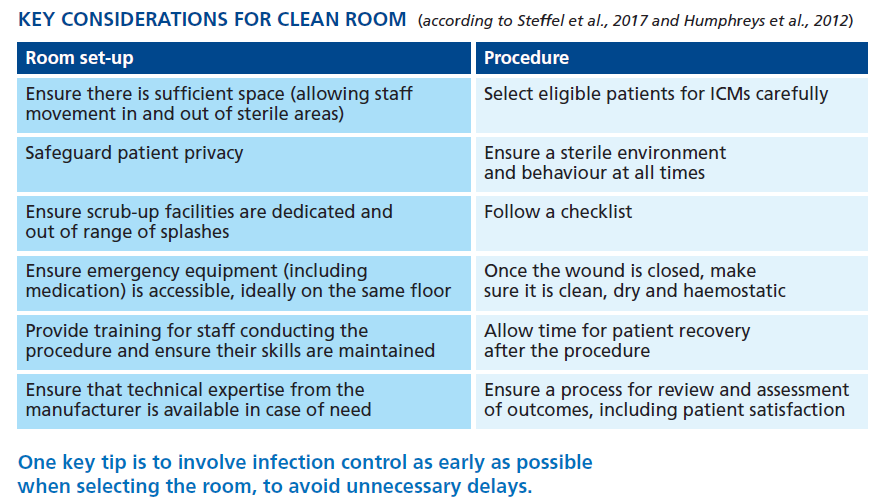
There’s no need for an operating theatre or a cath lab to implant the devices. Any available procedure room will work – as long as it has a sink to scrub up in, some privacy (perhaps in the shape of a door or curtain) and a bed or trolley for the patient to lie on while the implant is inserted (Steffel et al., 2017; Wong et al., 2016; Humphreys et al, 2012). Additional important considerations are listed in the table below.
Who will implant the device?
Traditionally, ICMs have been implanted by cardiologists or stroke physicians but thanks to the advances in technology with the Reveal Linq and insertable cardiac monitors, there are different people in the team who can implant the devices. In fact, there can be considerable financial and workload savings and staff development opportunities by looking elsewhere for implanters – with nurses and physiologists ideal choices.
Physiologists are able to program the device to CareLink once implanted, so some centres train them to perform the implant procedure as well. Nurses can also be trained both to implant and program the device. Or a combination of both can work well.
A major advantage of employing nurses for this role is the pathways already established for consent, prescribing and follow-up that are available to them, but not necessarily familiar to physiologists. If nurses can conduct the procedure, this should reduce the input required from clinicians: a nurse prescriber is able to conduct follow-up and prescribe antibiotics and anticoagulants as required. However, depending on staff availability, using the skills of both specialists could be an advantage.
Of course, it is not essential to delegate this work to other medical professionals – some centres are considering ‘one-stop’ ESUS clinics where stroke physicians can both assess the patient and insert the ICM in a clean area. However, this does depend on the timeliness of investigations.
How will the implanting process take place?
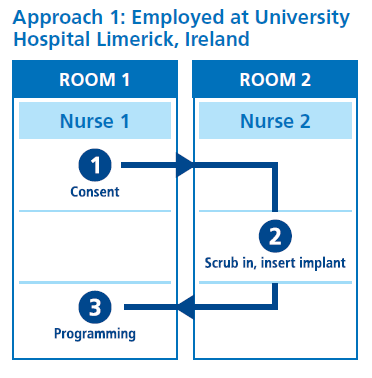
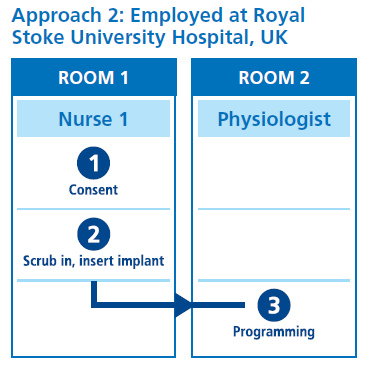
The process involves four key steps, outlined in Box 1. Implantation generally takes around 10 minutes, whereas programming the device can take up to 35 minutes – so a two-room strategy is advisable to optimise efficiency.
Two possible approaches are shown in the figure below, which both use a two-room strategy. In Approach 1, the patient is consented in the first room, moves to the second for implant insertion, then back to the first room for programming. In Approach 2, the patient is consented and has the device implanted in the first room before moving to a second room for programming (in this case by a physiologist). Alternative approaches could also be possible, dependent on the resources and staffing in the Trust.
When should the procedure be scheduled?
You should carefully consider when to schedule implants (and explants). As a diagnostic device, the ICM is subject to a six-week deadline from referral – to avoid breaching this and to keep waiting lists down, we recommend a weekly or two-weekly regular clinic for elective implants. (Inpatients can be scheduled separately, depending on their needs.)
When physiologists are involved in the programming step, scheduling implants (which require programming) on one day of the week and explants (which do not) on another can optimise efficiency and staff workloads.
What other points should be considered?
Attracting and training staff
The prospect of nurses to implanting ICMs presents an appealing development opportunity and a chance to climb the NHS pay bands, so we have not found attracting staff to be an issue. Training, too, is not especially onerous: official training can be provided by manufacturers, lasting around one day, combined with on-the-job shadowing that can vary in duration, depending on the comfort levels of the consultant and nurse being trained.
Training a nurse or physiologist for the first time naturally requires the greatest allocation of time and effort, but once the first training is completed you have all the necessary paperwork and systems in place to train others. Not only that, but once a few staff have been trained, they can support with teaching and mentoring. Additional efficiencies can be found by sharing training materials and experiences between Trusts, including inviting members of other Trusts to spend time observing the protocols and procedures.
Governance
A Standard Operating Procedure (SOP) will need to be drawn up, outlining the process for implantation, how to consent patients and available contacts for back-up in case of problems. This should be developed collaboratively by consultants and nursing management, before being taken on to the Governance Forum and beyond. We especially recommend close collaboration and coordination between the cardiology and stroke units to clearly delineate roles and responsibilities.
Each member of staff should be trained once on the SOP, then signed off – and again, to minimise duplication of work, we recommend sharing previously developed paperwork between Trusts as a source of inspiration and guidance.
Setting up a process for inserting ICMs need not be daunting. With the right setup and protocols in place, the procedure can be carried out swiftly, efficiently and frequently, with the involvement of nurses and/or physiologists freeing up medical resources to be deployed elsewhere.
References
-
Humphreys H, et al., 2012. Guidelines on the facilities required for minor surgical procedures and minimal access interventions. J Hosp Infect 80:103e109.
-
Steffel J, et al., 2017. Insertion of miniaturized cardiac monitors outside the catheter operating room: experience and practical advice. Europace 19:1624–1629.
-
Wong GR, et al., 2016. Feasibility and safety of Reveal LINQ insertion in a sterile procedure room versus electrophysiology laboratory. Int J Cardiol 223:13–17.
6. Monitoring, follow-up and aftercare of patients post implant
The ongoing monitoring of ICMs is a major consideration in establishing a service. It may take a number of months for the device to detect AF, and in this time the patient will most likely be discharged to primary care. Effective communications between secondary and primary care and clarity on roles and responsibilities is essential to make sure everyone is clear on what happens if AF is detected.
Monitoring ICM data
ICMs and other cardiac devices produce a huge amount of data. These days, more and more patients are being monitored remotely. The COVID-19 situation has encouraged cardiology services to embrace remote monitoring in even more patients (for example, patients with pacemakers), so this increase in data is likely to have an impact on the team’s workload.
While it’s important that data are reviewed carefully, this task doesn’t necessarily require a medic. Technicians can be trained to review data from the monitor and to know how to spot the ‘red flags’ that require investigation. Depending on the number of ICMs to be monitored, the size of the monitoring team may need to grow.
Some hospitals can manage the additional data and monitoring that a ICM service for stroke will produce within existing workflows. However, having a back-up plan is essential in case the workload becomes unmanageable or staff changes make it challenging. With protocols in place, data review can be provided via a remote service which essentially triages the data and alerts the clinical team to red-flag events.
The boxes below illustrate some options for management of this process.
Case Study
Managing monitoring in-house, with back-up support as an option
At Southend University Hospital, data monitoring is performed in-house by specially trained cardiac technicians. However, in case of overload, backup support is being considered.
Following an initial in-house check at 4 weeks, the team performs a regular 3-monthly review via remote monitoring, with a face-to-face visit at 1.5 years (halfway through the life of the device). Patient requests for further face-to-face consultations are accommodated in order to ensure the patient is reassured.
Case Study
FOCUSON™, monitoring and triage service
FOCUSON is a monitoring and triaging service provided by Medtronic, with the aim of helping hospital teams to save time and resource. Data from patients with Medtronic ICMs are uploaded to the CareLink™ Network, and the FOCUSON team monitors and triages the data according to hospital preferences. Where clinically relevant events are identified, the relevant hospital clinical teams are promptly alerted via telephone, email and the FOCUSON Platform.
More information is available at:
https://europe.medtronic.com/xd-en/health careprofessionals0/focuson-for-cardiology.html
While spotting and escalating the potential red flags is perfectly possible for a technician, clinical interpretation of these data should be performed by a cardiologist. When we insert a ICM we’re primarily looking for signs of AF, but the device could also pick up other conditions requiring management, such as runs of paroxysmal ventricular tachycardia, bradycardia or heart block. It’s important that roles and responsibilities are clear in anticipation of a situation like this: what triggers referral to the stroke pathway, and what to cardiology? In each case, how should the referral be implemented? What timescale for action is acceptable once an issue has been found? These should be considered when the pathway is developed (see chapter 3).
Communication and collaboration between secondary and primary care
If AF is not detected in while a patient is in the hospital, they are generally discharged with a ‘prevention prescription’ of antiplatelet therapy, blood pressure medication and statins. Anticoagulants are not prescribed without evidence of AF – however, if an implanted cardiac monitor subsequently shows AF, it is important that this is started quickly.
It is essential that when you set up your pathway for ICM in cryptogenic stroke, you consider what happens in primary care and involve GP representation. There may be specific local pathways relating to DOACs or certain drugs that are on the primary care formulary. It’s important that a discussion takes place with a local primary care representative when the pathway is being set up in order to ensure all parties are happy with the next steps. What is most essential is that everyone is clear on what happens if the ICM picks up AF to make sure that the patient is seen quickly and a DOAC is started as soon as possible.
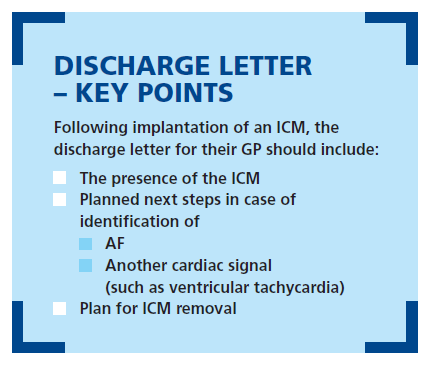
Importantly, the discharge letter for each patient must include the right information about the ICM for their GP to provide appropriate aftercare. These devices will be monitoring the patient’s heart for up to 3 years, and are sometimes not removed even after the battery has expired – so it’s essential that everyone is informed of the presence of the ICM and aware of their roles and responsibilities, along with next steps if a signal is found. If AF is spotted, the GP will need to be contacted and anticoagulation therapy initiated. If an alternate abnormal electrical rhythm is found the pathway and discharge letter should be clear on how the patient will be seen by a cardiologist for assessment and appropriate treatment.
Crucially, the GP must be aware that the ICM has been implanted in the first place. Aftercare should include removal of the ICM, either after battery depletion or after patient death: either way, the plan should be clear.
Finally, information is also important for patients themselves. This will be a worrying time for them: it’s important that each patient understands what the implant is, how it works, why it has been inserted and what it could detect. It goes without saying that the patient should also understand the planned next steps in case of signal detection, and the plan for eventual removal (or not) of the device.
JOIN VIRTUAL MEETING BELOW TO LEARN MORE:
THE RIGHT APPROACH FOR AF DETECTION AND SECONDARY PREVENTION
12 May, 2-5pm GMT
Faculty:
Professor Gregory Y.H. Lip, Professor Jesse Dawson, Professor Klaus Witte, Professor Nikhil Patel, Dr Paul Guyler, Dr Paul Cotter, Advanced Nurse Practitioner Imelda Noone & Advanced Nurse Practitioner Nora Cunningham
To ensure we make optimal use of time please register and access the pre-learning materials ahead of the day.
The course and content are CPD accredited.