Atrial fibrillation (AF) is the most common abnormal heart rhythm and is associated with a fivefold increased risk of stroke, and therefore represents a significant global healthcare burden. Recently, the therapeutic options for AF have increased, following the availability of non-vitamin K antagonist oral anticoagulants (NOACs).1,2 In order to discuss some of the most important issues regarding NOACs, a roundtable event was held in London on 12 November 2014, with the following objectives: to review the established evidence base for non-vitamin K antagonist (VKA) thromboprophylaxis in AF; to consider recent and emerging data on NOACs in AF trials and registries; to discuss on-going NOAC research in patients with AF; to identify and refine the indications for specific NOACs in AF; and to establish the nature of further NOAC research needed for new AF indications. The expert panel was chaired by John Camm (St George’s, University of London, UK) and also included Marco Alings (Amphia Ziekenhuis, Julius Clinical Research Julius Center, UMC Utrecht, the Netherlands), Raffaele De Caterina (G d’Annunzio University-Chieti and G Monasterio Foundation-Pisa, Italy), Paulus Kirchhof (University of Birmingham, UK), Jean-Yves Le Heuzey (Georges Pompidou Hospital, René Descartes University, Paris, France), and Freek Verheugt (Onze Lieve Vrouwe Gasthuis, Amsterdam, the Netherlands)
Real-world versus Clinical Trial Data with NOACs
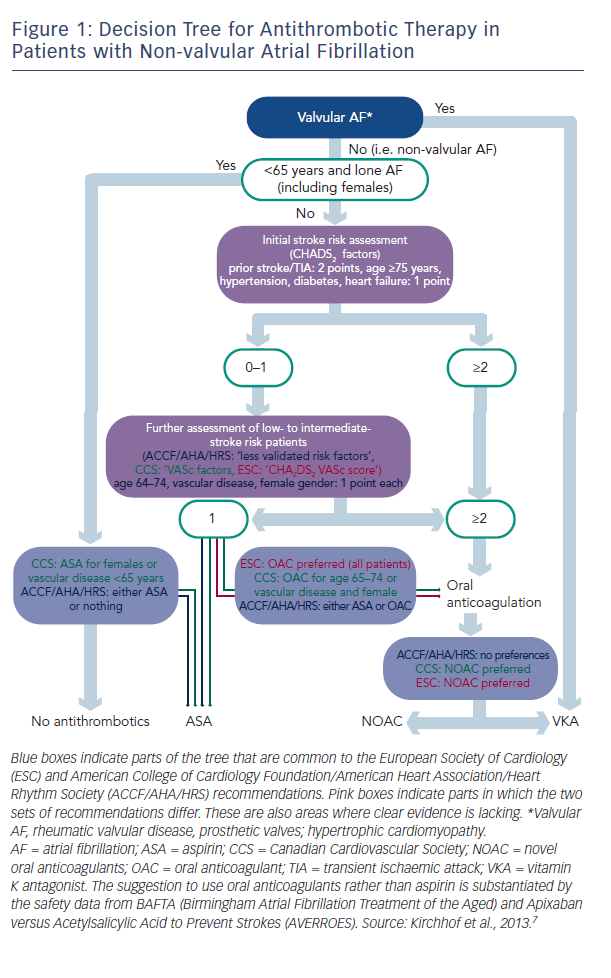
There are two types of data available to inform the use of NOACs: that from large clinical trials and that from registries, and these data are of different quality. Professor Kirchhof discussed the current treatment paradigms in valvular AF and presented a decision tree for antithrombotic therapy in patients with non-valvular AF, which combines the recommendations of current European Society of Cardiology (ESC) guidelines and those of the American College of Cardiology Foundation (ACCF)/American Heart Association (AHA)/Heart Rhythm Society (HRS) (see Figure 1).3 These recommendations show that in valvular AF, VKAs are the preferred treatment option. In non-valvular AF, the US recommendations give no preference between VKAs and NOACs, while the European and Canadian guidelines state that NOACs are the preferred option.
Although widely used, aspirin is not recommended: clinical trial data suggest that warfarin reduces the risk of major stroke, arterial embolism and other intracranial haemorrhages compared with aspirin in elderly patients.4 In addition, in a comparison of apixaban and aspirin in 5,599 patients with AF who were at increased risk of stroke and for whom VKA therapy was unsuitable, the same amount of major bleeding seen in both, but the study was terminated early because of a clear benefit in favour of apixaban.5 Lifelong oral anticoagulation is indicated for all AF patients with two or more CHA2DS2VASc (chronic heart failure, hypertension, age >65, diabetes, prior stroke or transient ischaemic attack [TIA], vascular disease, sex category) factors. Patients with only one of these risk factors may also benefit from oral anticoagulation. Almost everyone with AF is eligible for oral anticoagulation.6 In valvular AF, VKAs are the preferred treatment option. In non-valvular AF, the US guidelines give no preference between VKAs and NOACs, while the European and Canadian guidelines state that NOACs are the preferred option.7
Four landmark trials of NOACs have formed the basis for guidelines: RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy; dabigatran),8 ROCKET AF (Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation),9 ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation),10 and ENGAGE AF-TIMI (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation – Thrombolysis in Myocardial Infarction 48; edoxaban).11 A recent meta-analysis of all 71,683 participants included in these trials showed that NOACs had a favourable risk–benefit profile, with significant reductions in haemorrhagic stroke and intracranial haemorrhage, but increased gastrointestinal bleeding.12 In addition, a 10 % mortality benefit was seen with NOACs.
In terms of real-world data, the GARFIELD (Global Anticoagulant Registry in the FIELD) study, an observational study of patients newly diagnosed with non-valvular AF, found that NOACs are not being used according to stroke risk scores and guidelines, with overuse in patients at low risk and underuse in those at high risk of stroke.13 Recent 1-year data from the EORP-AF Pilot (EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase) suggest the majority of patients (84 %) who were taking a VKA at baseline, remain on a VKA afterwards.
Likewise, patients initiated on a NOAC were likely to remain on a NOAC. Encouragingly, many patients who had received antiplatelet therapies have switched to OACs after a year.14 Another registry study, PREFER in AF (Prevention of Thromboembolic Events – European Registry in Atrial Fibrillation) found that NOACs constitute 5–10 % of use.15
The panel discussed the value of registries. It was considered that they were necessary as they generate useful data next to the phase III clinical trial data. Many patients do not participate in clinical trials because they do not meet the exclusion criteria or do not want to participate in a trial. The data generated by clinical trials can be to some extent artificial because, for example, drug intake is monitored for each patient. Furthermore, not all side-effects are picked up in phase III trials as several 100,000 people need to be exposed to the drug to reveal certain rare effects. However, registries are rarely controlled properly in terms of randomisation, therefore it can be difficult to put the results into perspective. Professor Le Heuzey considered that registries generate valuable data e.g. GARFIELD shows a lower rate of anticoagulation than in clinical trials. Registries give us an idea of the different settings in which NOACs are being used, the uptake of NOAC therapy and improved safety information, but efficacy is hard to assess. We need to know in which settings registries are run (e.g. by cardiologists or GPs) and what the inclusion criteria are for registries in order to interpret them correctly, according to Professor De Caterina. They can also help inform why data arising from clinical trials are not put into practice. For example, based on clinical trial data, the use of aspirin is discouraged in AF patients and yet registry data tell us that aspirin is still in widespread use. The PREFER registry showed that doctors treat vascular disease and AF as two independent entities so therefore when these conditions coexist, two different drugs are used. In order to maximise their usefulness, registries need more outcome data, as can be generated by pragmatic trials. NOACs perform as well in real world registries as in clinical trials but VKAs perform worse in real world data. So more trials in daily use setting are needed. In summary, the panel concluded that registry data are extremely helpful and complementary to clinical trial data and in many instances confirmatory, particularly in terms of safety.
Once or Twice-daily NOAC Therapy – Theory and Practice
Professor Camm began by highlighting the decisions that need to be made when developing NOACs. Among the four available NOACs, two are taken once daily and two are taken twice daily. Since the half-life of each NOAC is around 12 h, the decision regarding whether to administer once or twice daily is a borderline one. Data from the EUropean Patient Survey in Atrial Fibrillation (EUPS-AF) regarding patient preferences for taking medication once daily or twice daily, show that, overall, 81 % stated a clear preference for once daily anticoagulation.16 However, the pharmacokinetics of these drugs is interesting. A drug given once daily has a much lower trough level after 24 h than a drug administered twice daily. If a once daily drug is missed, a very low trough level results, whereas the impact of missing a twice daily drug is of less concern since the patient remains presumably better anticoagulated. As a result, there is considerable debate about whether missing a single dose is more hazardous when a drug is given once or twice a day. However, in a phase II dose-finding study of edoxaban, the bleeding frequency was higher in the 30 mg twice daily group than in the 60 mg once daily group.17 Although studies with other NOACs did not show this effect, this intriguing finding has led physicians to question whether a once daily or twice daily regimen is preferable for NOACs.
Surveys have found that once daily regimens are associated with greater adherence than twice daily regimens: a database analysis of cardiovascular patients found that adherence to all agents was 16 % greater for once daily versus twice daily. (p<0.01).18 Another study found that nonvalvular AF patients treated with once daily dosing regimens for chronic medications were associated with approximately 26 % higher likelihood of adherence compared with subjects on twice daily regimens.19 Starting patients on rivaroxaban may decrease the number of total ischaemic strokes relative to warfarin treatment.20 It has been argued that if a patient is already taking medication on a twice daily basis, as many CV patients are, then giving another drug twice daily does not produce any issues, whereas others point out that once daily is more convenient, as most people can generally be in the same place once a day but not twice.
The panel reached no consensus on this controversial matter. In the experience of Professor Le Heuzey, patients who have previously taken once daily VKA have expressed reluctance to switch to a twice daily NOAC. Switching drugs from once daily to twice daily is more of an issue than initiation on a twice daily regimen. Other panellists considered that adherence to NOACs is essential and finding ways to improve adherence is a more important challenge than deciding whether once daily or twice daily dosing is better. Professor Kirchhof underlined the consistent signal from various phase III trials of less intracerebral haemorrhage with NOACs in comparison with VKA, irrespective of dosages, even the total dosages in some cases. In addition to pharmacokinetics, practical factors such as tablet size contribute to the totality of drug exposure. In terms of electrophysiology, missing a once-daily dose before a procedure could result in patients being suboptimally anticoagulated, e.g. for an ablation procedure, but whether once or twice a day, adherence in general remains a difficult matter. The panel agreed that it requires further study. Professor Camm concluded by stating that adherence is the key mediator between medical practice and patient outcomes.
Spot Checks or Monitoring of NOACs for Anticoagulation Status
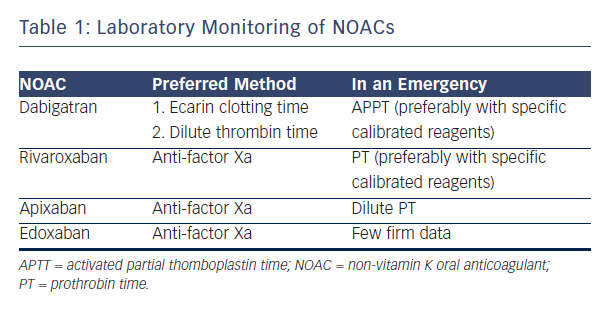
NOACs are considered controversial because of the risk of excess bleeding. It has therefore been suggested that blood levels of NOACs should be monitored. Professor Verheugt suggested that the lack of necessity for blood monitoring in NOACs is an advantage, but acknowledged that it is occasionally necessary to know blood levels and considered how NOACs could be monitored. Dabigatran, the most well-established NOAC, blocks the activity of thrombin. The activated partial thromboplastin time (aPTT) may be used, but numerous assays for aPTT exist.21 The situation with factor Xa blockers is more complex. For rivaroxaban, prothrombin time (PT) may be used as a screening test to assess the risk of bleeding. This test is available at all times in all hospitals but the prolongation time is not impressive.22 The optimal way to measure anti-factor Xa (anti-fXa) levels is a standard laboratory anti-fXa assay: apixaban levels and anti-fXa activity show a good correlation.23 However this test is complex, expensive and not available in most institutions 24 hours a day. A systematic review showed the usefulness of standard tests: dabigatran, rivaroxaban and apixaban show variable results on coagulation assays and linearity is not seen in the entire therapeutic range.24 In conclusion, aPTT is the only useful monitoring technique for dabigatran. For rivaroxaban, the anti-fXa assay is preferred but, in an emergency room situation, PT may be used.
The anti-fXa assay is also preferred for apixaban but in an emergency, dilute PT may be used. For edoxaban, at the moment, limited data are available to advise on laboratory monitoring in an emergency scenario (see Table 1).25
The panellists discussed the problems arising from the fact that quantitative tests involve relatively expensive tests that are not available in most institutions at all times. They also discussed whether such monitoring is, in fact, necessary. Spot measurement is needed in certain situations, e.g. bleeding or preparation for surgery when the time of last drug intake is uncertain, but these are occasional situations only. The ability to react quickly to test results is compromised by the lack of experience of laboratory staff and, given the variable response of NOACs to standard tests, each test needs to be calibrated with the specific NOAC.
In terms of routine monitoring, the panel considered that one of the great advantages on the NOACs is the lack of need to monitor, allowing increased uptake of the agents among those who need them. Despite the likelihood that some patients have higher plasma levels of NOACS than others, e.g. because of compromised renal function, these do not necessarily translate into different clinical outcomes. However, pharmaceutical companies are coming under criticism for not having developed specific monitoring tests. In certain clinical situations, e.g. if a patient presents with a large ischaemic stroke, it may be useful to have a simple assay to determine whether that patient has taken their NOAC. In rare cases of severe bleeding, it may be useful to establish whether the NOAC caused the bleeding, but again, a sophisticated, quantitative assay is not needed; we merely need to know whether or not the NOAC has been taken, and assess renal function, which is simple and informative. The consensus of the panel that was that there was no need for routine monitoring or quantitative monitoring.
Antidotes or Reversibility Agents – Are they Needed?
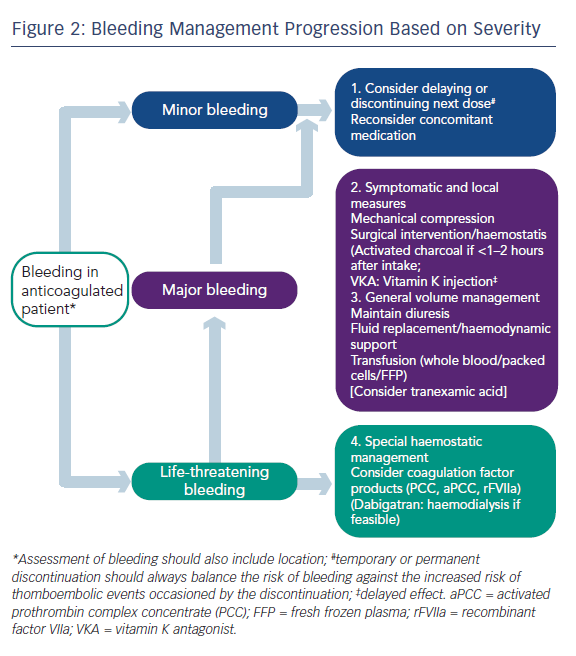
Professor Camm began by stating that whenever asked about NOACs, the matter of reversibility is always mentioned, because anticoagulant drugs may cause serious haemorrhage. At present, the management of bleeding can be largely managed by standard procedures such as pressure on the bleeding point, and in cases of major bleeding, replacement of blood volume, etc. (see Figure 2). However, reversal strategies may be needed in cases of life-threatening bleeding or if the action of the drug is long and needs to be antagonised in emergency situations.26 It should be remembered that the half-life of these drugs is relatively short (around 12 hours) and the anticoagulant effect of the drug will diminish much more quickly than that of warfarin, which often requires much more active intervention. While specific antidotes would be desirable, the major clinical trials of NOACs took place without the availability of antidotes.
Specific reversal agents for NOACs are currently under clinical investigation. These include andexanet alfa (Portola Pharmaceuticals) administered alone or with factor Xa (fXa) inhibitors, (phase II completed27,28 and phase III trials recruiting),29,30 idarucizumab, (Boehringer Ingelheim) administered alone or with dabigatran (phase I competed31 and phase III recruiting)32 and PER977 (aripazine, Perosphere Inc.) administered alone or with edoxaban (phase I study completed).33 Andexanet alfa is a modified, recombinant human fXa molecule that is catalytically inactive but retains high-affinity binding to direct fXa inhibitors and is a universal factor Xa inhibitor reversal agent. Phase II studies in healthy volunteers have provided rapid, sustained and dose-related reversal of apixaban and rivaroxaban.28 Idarucizumab is a fully humanised monoclonal antibody fragment (FAb) against dabigatran.34 In vitro characterisation and PK analyses showed that the Fab has very tight binding affinity to dabigatran, with rapid onset and slow offset.35 Idarucizumab had no effect on bleeding in rats receiving VKA.
The phase III trial will evaluate the reversal of dabigatran by intravenous administration of idarucizumab in patients who have uncontrolled bleeding or require emergency surgery or procedures.32 Aripazine is a small synthetic molecule with activity against all NOACs and is in early stage clinical development.33
In the panel discussion, Professor Verheugt mentioned first that the NOACs – for which there is no antidote as yet – showed in their trials a reduction in fatal bleeding. He stated that it is important that reversibility agents are effective in bleeding situations – while warfarin can be reversed, this does not stop the bleeding – and it is difficult to conduct clinical trials on bleeding patients. It was also pointed out by Prof Kirchhoff that in a comparison between apixaban and aspirin, the rate of major bleeds was the same despite presumably equal plasma coagulation in the aspirin group.5 He also stated that we [doctors] want to have control about the harm we cause ourselves. Prevention of bleeding is easier than cure. The antidote will not stop the accident and haemostasis will still be required. Availability of antidotes is, however, a serious concern and the availability of an antidote may make NOACs more acceptable to patients and general practitioners, according to Professor Le Heuzey.
The consensus of the panel was that transferring knowledge to patients and the lay press is more important than the availability of an antidote. The panellists would like to have an inexpensive antidote for patient reassurance, even if in clinical practice it might not be much needed, but it should have a long shelf life as it would be used infrequently. Prevention of bleeding is more important than using an antidote, and the short half-lives of NOACs ensures a relatively low rate of major bleeding episodes.
NOACs and Dual Antiplatelet Therapy
The use of NOACs with dual antiplatelet therapy (DAPT) presents a therapeutic dilemma. There is uncertainty over the optimal antithrombotic management strategy for patients with AF presenting with an acute coronary syndrome and/or undergoing percutaneous coronary intervention (PCI)/stenting.36 Professor De Caterina showed that 8–10 % of AF patients of cardiology departments develop ACS or need elective stent implantation and 15–20 % of post ACS or electively stented patients develop AF and are therefore candidates for anticoagulation.36 The use of DAPT in patients with AF after coronary stenting is essential to prevent stent thrombosis and is supported by a large body of clinical trial data; however, the use of OACs is needed to reduce the risk of stroke, and this therapeutic entity has been well-evaluated in many trials.
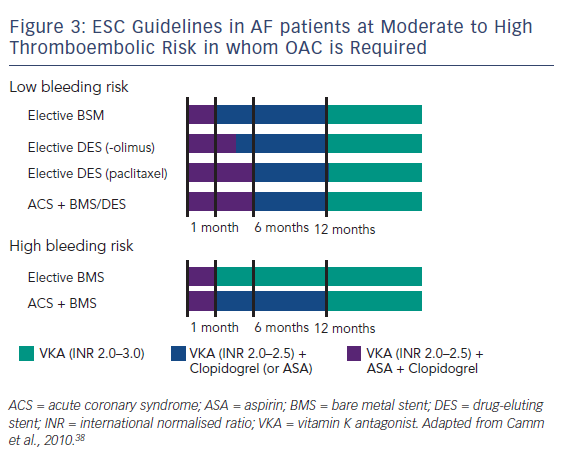
When used together, these therapies confer a major bleeding risk. In a Danish cohort study using national registries to identify over 82,000 patients with AF during the period 1997 to 2006 taking post-hospital therapy of warfarin, aspirin, clopidogrel and combinations of these drugs, 11.4 % developed a nonfatal or fatal bleeding over a mean 3.3 years’ follow-up, with those on triple therapy having an incidence rate of 10–13 % per patient year.37 It is therefore vital to establish an equilibrium between the risk of thrombosis (stent thrombosis and stroke) and the risk of bleeding. The ESC guidelines have stratified patients into low- and high-risk groups to minimise the time of overlap of drugs. The time of triple therapy should be as short as possible; a maximum of 1 month is recommended for the combined use of aspirin and clopidogrel, on the background of a vitamin K antagonist (VKA), with elective stenting with bare-metal stents (BMS). With drug-eluting stents (DES) in the setting of elective PCI, a maximum of 6 months and, for more recent stents, 3 months is suggested, then continuing with one single antiplatelet agent and the VKA. In the case of ACS a triple therapy overlap is advised for 6 months. The overlap should, however, be shorter in patients at high bleeding risk, in whom BMS are recommended anyhow, and in such cases the continuation of antithrombotic therapy with a VKA only is recommended after the first month. In case of patients at high bleeding risk having received stenting (usually BMS) because of an ACS, triple therapy is still restricted to 1 month, but continuing a VKA with the addition of one antiplatelet agent (clopidogrel or aspirin) up to 1 year (see Figure 3).38 A consensus document of the AHA is more complex and was not discussed here.39 However, these guidelines only cover triple therapy involving VKAs; triple therapy involving NOACs is still under investigation.
Several trials are ongoing in patients with AF and ACS and/or coronary stents. The PIONEER trial (a study exploring two strategies of rivaroxaban – 15 mg once daily + clopidogrel; 2.5 mg twice daily + DAPT for the initial 1, 6 or 12 months – versus the ‘classic’ triple therapy with a VKA + aspirin + clopidogrel in patients with AF who undergo a PCI),40 will enrol 21,000 participants. The RE-DUAL-PCI (Evaluation of Dual Therapy With Dabigatran vs Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting) trial will evaluate dabigatran (110 mg twice daily) + a single APT, dabigatran 150 mg twice daily + single APT and warfarin and DAPT.41 All these trials have limitations in that more than one component of the comparator ‘cocktail’ – a VKA + aspirin + clopidogrel – is being changed at a time, and the final interpretation of the data may prove difficult.
A panel discussion ensued about the need to use aspirin post-PCI. The panellists agreed that there was an excess co-prescription of these drugs and that it may be safe to use single APT with NOACs. Clopidogrel is preferred unless there is a definite stent thrombosis, according to Professor Kirchhof, in his institution, as newer agents have not been tested in patients who are anticoagulated. Patients in the RE-LY trial underwent stenting and, while bleeding rates increased, there was no difference in outcomes between warfarin and dabigatran. When performing interventions in anticoagulated patients presenting with ACS, every possible effort should be taken to prevent bleeding, e.g. a radial rather than femoral approach. The new bio-absorbable stents should minimise the need for long-term triple therapy. In conclusion, the addition of NOACs to APT may help prevent ischaemic events and stent thrombosis, but this needs testing in a clinical trial setting. It is important to balance the antithrombotic and anticoagulant effect of the different agents, but more clinical trial data are needed.
NOAC Treatment in Chronic Renal Impairment
Professor Le Heuzey started by describing the absorption and metabolism of NOACs. All NOACs are partially eliminated by the kidneys. The renal elimination of NOACs varies considerably: dabigatran 80%, apixaban 25 %, rivaroxaban 35 % and edoxaban 50 %, and therefore different NOACs have different levels of contraindications.42 Practical recommendations have been proposed for the use of NOACs in chronic kidney disease (see Table 2); dabigatran is contraindicated if creatinine clearance (CrCl) is lower than 30 ml/min, apixaban and rivaroxaban if lower than 15 ml/min (no data available for edoxaban at time of recording).1,2 Bioavailability also differs according to the drug: for dabigatran, 3–7 %; for edoxaban 50 %, for edoxaban 62 %, and for rivaroxaban 66–100 %. Therefore, different dosages are recommended in suboptimal renal function. The age of the patient should also be taken into account because elderly patients often have decreased renal clearance.
Guidance is also available regarding when to stop NOACs before a planned surgical intervention. The duration of action of the drug is directly related to CrCl: with a normal renal clearance NOACs can be stopped 24–48 hours before a planned surgical intervention, but for example with a CrCl of 30-50 ml/min, the effect of dabigatran can persist for up to 96 h. The risk of bleeding associated with the intervention should of course also be taken into account. The proposal of EHRA is that renal function should be monitored regularly in patients taking NOACs and the dose adapted accordingly. Renal function should be monitored at the following intervals in patients with chronic kidney disease: yearly with a CrCl ≥60 ml/min; 6-monthly for elderly (>75 years) or frail patients on dabigatran (CrCl 30–60 ml/min); and 3 monthly when CrCl ≤30 ml/min.
In the panel discussion, Professor De Caterina considered that NOAC therapy should not be used in patients with CrCl ≤30 ml/min. A traffic light analogy was suggested for advising doctors, with a red light at CrCl ≤30 ml/min, yellow at 30–50 ml/min and green ≥ 50 ml/min. However, following the availability of drugs with lower renal clearance, this might change in the future, especially if dosages are carefully tailored to the individual.
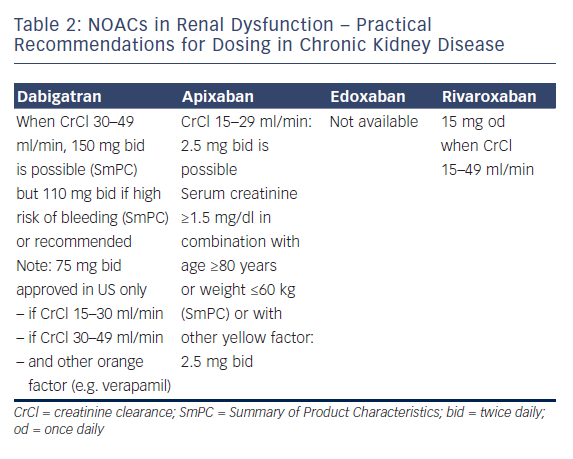
At adjusted dosages, the NOACs have shown preserved efficacy in patients with impaired renal function, whereas there is little evidence for the efficacy of warfarin in these patients. The choice of drug should be influenced by renal function; while some panel members still prescribe warfarin to patients with CrCl 30–50 ml/min, all concede that NOACs might be preferable. Regular CrCl monitoring is essential. The panellists consider that ‘NOAC outpatient-clinics’ in some countries are well equipped to handle the treatment decisions, follow-up and paperwork involved with prescribing NOACs, not only in patients with renal impairment. Despite these restrictions, in most phase III trials, patients with renal impairment derived considerable benefit from NOACs. In conclusion, as far as renal function is concerned with regards to NOACs, renal function must be known before choosing a drug, before choosing the dose, in follow-up because renal function will change, and when planning, e.g. a surgical intervention. Renal function is important with regards to patients taking NOACs.
How to Choose Between NOACs
In order to choose between NOACs, Dr Alings started by highlighting the differences between the guidelines: the ESC AF guidelines discuss when NOACs should be used1 and in addition, the EHRA practical guide suggests how the anticoagulation should be achieved with NOACs.2 According to the ESC guidelines, a CHA2DS2VASc ≥1 indicates the use of an OAC. Several factors may influence treatment choice. The renal clearance varies considerably among the NOACs. In clinical trials of NOACs compared with VKAs, drugs were dose adjusted according to renal function.
Acknowledging differences between clinical trials, in over 70,000 patients studied, all NOACs proved non-inferior to warfarin, and some were found to be superior for prevention of stroke and bleeding.8–11 In terms of stroke risk, the risk of haemorrhagic stroke accounts for the reduced risk observed in these trials. In terms of bleeding risk, ENGAGE, ARISTOTLE and RE-LY (at the lower dose) showed significantly reduced risk of major bleeding compared with warfarin. To summarise the impressive results of the NOAC trials: major reductions in intracranial bleeding were seen compared with warfarin, with RE-LY, ARISTOTLE and ENGAGE being associated with the greatest mortality benefits.
In conclusion, where OACs are indicated, NOACs should be considered rather than dose-adjusted VKA. The choice of NOAC may be influenced by concomitant disease, such as renal function and a prior gastrointestinal bleed, and concomitant drugs (e.g. P-glycoprotein inhibitors). In patients with a low bleeding risk, it is probably best to select the NOAC with the best reduction in ischaemic stroke. In patients with a high bleeding risk, the NOAC with the best reduction in major bleeding compared with VKA is probably the best option. Patient preference to once daily versus twice daily regimens may also influence the choice of NOAC. Finally, regional or national availability and reimbursement of the individual agents may affect treatment choice.
The panel discussion emphasised the fact that the methodologies of the NOAC clinical trials differed, and are therefore not directly comparable. However, the outcomes were highly consistent across trials. Professor Kirchhof stated that meta-analyses of these trials have found remarkable similarities in reduction in intracranial haemorrhage, mortality benefits and reduction in major bleeding. However, statistical significance depends on the number of events observed, rather than the compound studied; this is important when extrapolating clinical trial results to different patient populations. In terms of efficacy, valid comparisons cannot be made between NOACs given the available data. Factors such as pharmacokinetics, dosage frequency and capsule size are much more important in clinical practice when making the choice for a NOAC. Professor Le Heuzey suggested that the bleeding risk is useful in selecting the best NOAC for a patient but cannot be easily predicted. For many general practitioners, detailed knowledge of each NOAC is not necessary; the best drug might be the one with which they have most experience and they should not try to differentiate. Professor De Caterina reminded the panel that efficacy and safety should not be considered in isolation: the compound parameter of efficacy and safety, the net clinical benefit, is an important evaluation. He highlighted the fact that, in order to optimise treatment, it is important to remember that not all risks have the same prognostic implication, but, for example, haemorrhagic stroke has a worse prognosis than ischaemic stroke. The only way to determine the optimum NOAC is to perform a head-to-head-comparison but is questionable whether this is feasible. The panel concluded that at this moment each patient should be assessed on a case-by-case basis, carefully looking at the risk factors.
Summary and Take Home notes
The use of NOACs in patients with AF is associated with benefits in terms of mortality and major bleeds, especially intracranial haemorrhage, but concerns persist regarding their use. This roundtable discussion has addressed several important matters, including the importance of registry data, dosage frequency, the monitoring of blood levels of NOACs and the development of antidotes, the use of NOACs in specific patients and the optimum choices of NOACs. While there remains a need for further clinical trial data, the expert panel concluded that, with the exception of patients with severe renal impairment, NOACs are safe and effective in the large majority of patients