The origin of idiopathic ventricular tachycardia (VT) or symptomatic premature ventricular contractions (PVCs) from papillary muscle (PM) was first described in 2008 as a distinct clinical syndrome by a group from Birmingham, Alabama, US.1 Out of 290 patients ablated for idiopathic VT or symptomatic PVCs, seven patients were recognised who had the ablation site at the base of the posteromedial PM. The ECG pattern was characteristic, showing a right bundle branch block and superior axis deviation. The main mechanism of these arrhythmias was identified as a non-reentrant mechanism (automaticity or triggered activity). Activation mapping revealed the source of focal activity at the base of the posterior PM. All patients were free of PVCs or VT at 9 months follow-up.
One cannot dismiss the question why such an entity was not described earlier. One explanation could be that it is difficult to exactly locate the focus without direct visualisation with echocardiography and/or intracardiac echocardiography. The other reason could be that this arrhythmia was often misdiagnosed with fascicular VTs or with arrhythmias from the mitral annulus region. It is no surprise that the same group published another series of six cases of focal arrhythmias originating from the base or midportion of the anterior PM in the left ventricle (LV) 1 year later.2 Again, ECG showed typical morphology of a right bundle branch block with inferior axis deviation. Successful ablation required a cool tip or 8 mm tip catheter. Finally, in 2010, ventricular arrhythmias from the PM in the right ventricle (RV) were described by Crawford et al. in eight of 169 patients with idiopathic RV arrhythmias.3 The majority originated in the septal PM (n=8), while anterior and posterior were a less frequent source of focus (n=4 and n=3, respectively).
Focal sources of arrhythmias at the PMs were also described in patients after myocardial infarction. Interestingly, these patients presented with heterogeneous late gadolinium enhancement within the region of the PMs,4,5 and such PM substrate may even participate in reentrant VTs.
Even more recently, PMs were recognised to also be a source of focally triggered VF.6–8 In one report, the moderator band was identified as a source of focal trigger for VF.8 The other series described two patients with VF originating from the left posteromedial PM. However, four patients had VF from the RV, specifically from the posterolateral PM.7
Anatomical Considerations
In the LV, the PMs are part of the mitral valve apparatus. The anterolateral PM originates from the anterolateral LV wall, and provides chordae to the anterolateral half of the anterior and posterior mitral leaflets. The posteromedial PM originates from the inferoseptal LV wall, and provides chordae to the posteromedial half of both leaflets. Imaging studies revealed that both PMs vary significantly in size and shape. Online use of intracardiac echocardiography (ICE) revealed that PMs can have two heads.9
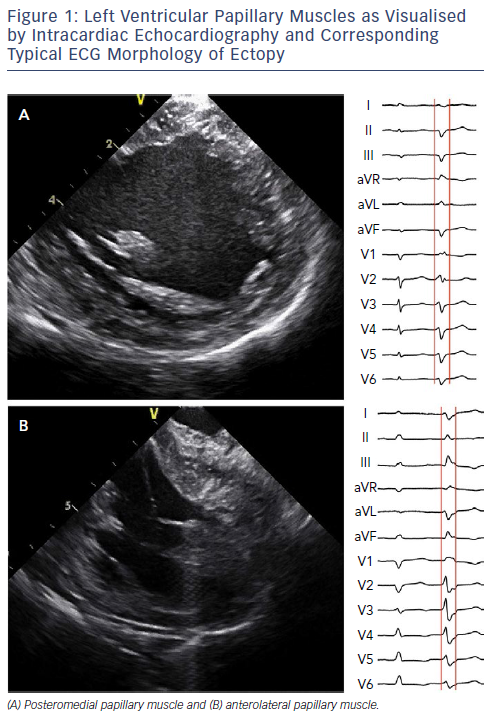
Our recent multicentre experience in 34 patients with idiopathic VT/PVC from PM included the detailed description of the anatomy of PMs using ICE.10 The anterolateral PM was shorter (23 ± 4 mm) than the posteromedial PM (28 ± 7 mm). In about one-third of patients, the PM was formed by two distinctly separate heads. Two-head PM can be considered as a normal anatomical variant (Figure 1).
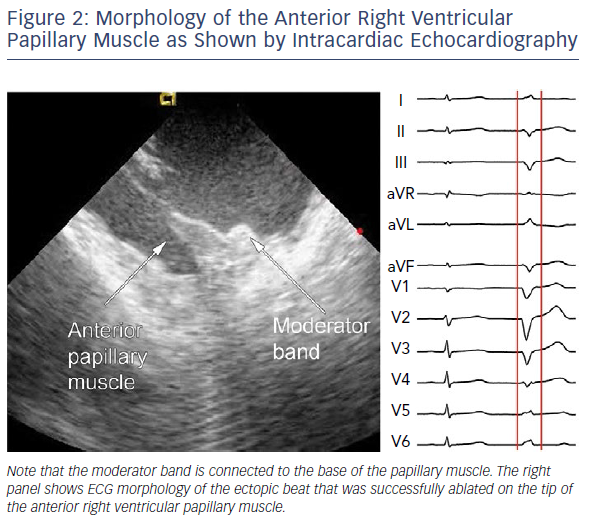
In the RV, the PMs are even more complex in arrangement.11 The moderator band is a prominent muscular trabeculation that crosses from the septum to the free wall of the RV and provides support to the anterior PM of the TV (Figure 2). It contains the right bundle branch of the conduction system and its terminal arborisation. The posterior PM provides chordae to the posterior and septal leaflets. The conus PM provides chordae to the septal leaflet of the valve.
From a histological point of view, the PMs contain ventricular myocytes and a rich subendocardial network of Purkinje fibers covered by a layer of endothelium. The ventricular myocardium and the Purkinje network are connected only at localised regions near the PM base, explaining why separate Purkinje potentials can be recorded here.
Differential Diagnosis
ECG
Since idiopathic LV arrhythmias arising from the PMs, left fascicles, and mitral annulus, and ventricular arrhythmias arising from the septal PM or RV outflow tract may have similar ECG morphologies, differential diagnosis based on ECG is very important. Arrhythmias originating from the LV have right bundle branch morphology in lead V1. The origin in the PMs can be suspected from the morphology of the QRS and from the location of the transition zone. Differentiation between anterolateral and posteromedial PM can be made using the axis in the frontal plane. Focus in posteromedial PM has a superior axis, which is similar to left posterior fascicular and or perimitral arrhythmias. Arrhythmias from the anterolateral PM have an inferior axis similar to arrhythmias from the left anterior fascicle and the anterior part of the mitral annulus (Figure 1).
Several studies suggested characteristics that can distinguish among the aforementioned arrhythmias.12,13 Narrower QRS (<130 ms) and rR’ pattern in V1 are characteristic of fascicular arrhythmias, as they use by definition the conduction system.12–14 In contrast, broad QRS and the absence of rR’ are features typically observed in PM arrhythmias. The recent algorithm based on this value suggests high sensitivity and specificity (approximately 90%). Arrhythmias from the mitral annulus are characterised by positive concordance in chest leads, as compared with PM arrhythmias with the transition zone between V3 and V4. This reflects the location of the base of the PM approximately in the middle of the long axis of the ventricle.
Other studies demonstrated that an R/S ratio <1 in lead V6 in the LV anterolateral region and QRS duration >160 ms in the posteroseptal region were the only reliable predictors for differentiating PM VTs from fascicular arrhythmias.13 Another sign to differentiate between PM and fascicular arrhythmia is Q waves in limb leads. Their presence in leads I, aVL or II, III and aVF suggests a fascicular origin.14
Ventricular arrhythmias from the RV PM are much less frequent than arrhythmias from the LV. In general, such arrhythmias have a left bundle branch block morphology and an rS or QS pattern in lead V1 (Figure 2). Among them, the focus in the septal PM may resemble the source in the RV outflow tract on ECG, especially when the focus is on the conus PM.11 Then, the QRS is positive from lead I to III. Some additional characteristics may favour an origin from the parahisian area,15 including prominent R waves in leads I and aVL, and a lower R wave amplitude in lead III than lead II. Other right-sided PM arrhythmias have a wider QRS complex with a notched appearance in the precordial leads. Posterior or anterior RV papillary muscles usually have a superior axis with a late R wave transition (>V4) as compared with septal PM arrhythmias, which often have an inferior axis and an earlier R wave transition.3,16
Finally, the discrepancy between leads II and III was proposed as a simple sign, suggesting an exit from a mid-cavitary structure.17 Discrepancy means a predominantly positive QRS complex in lead II with a negative QRS complex in lead III (positive/negative discordance, equivalent to a frontal axis of −30° to +30°) or the opposite finding (negative/positive discordance, equivalent to a frontal axis of +150° to +210°). The first scenario (i.e. positive/negative discordance) suggests the source from the LV septum or RV (either parahisian or moderator band/right septal PM), while the latter (i.e. negative/positive discordance) suggests the LV free wall (anterolateral PM). While negative/positive discordance is very specific for anterolateral PM, its sensitivity is low (most anterolateral PM foci have an inferior axis).
Clinical Parameters
Patients with arrhythmias from PM tend to be older compared with patients with fascicular or perimitral arrhythmias.12 They more often have coronary artery disease and some degree of LV dysfunction. They are frequently treated with beta-blockers, while calcium channel blockers are often used for fascicular arrhythmias.
Imaging Techniques
Echocardiography is a part of the diagnostic workup for every patient. In selected cases, cardiac magnetic resonance is used. Most idiopathic PM arrhythmias have normal findings both on echocardiography and cardiac magnetic resonance. However, late gadolinium may be detected in the PM in some idiopathic patients, corresponding with localised areas of low voltage.14 This finding is more frequent in patients with ischaemic heart disease.4 ICE may also reveal hyperdensities in PMs; however, no rigorous study has been performed to set criteria for the presence of a scar in PM.
ICE appears to be the most suitable for assessment of the exact location of the focus at PMs. Its role was already emphasised in 2010 by a group from the Mayo Clinic.18 In another study, 7% of 122 patients presented with ectopy from left-sided PMs as confirmed by ICE.14 Intraprocedural imaging also helped in differential diagnosis between PM-related arrhythmia and fascicular tachycardia. Our experience confirms this notion, as without online imaging it is difficult or even impossible to describe the exact location of the focus.
Electrophysiological Study
Regarding the mechanisms of arrhythmias from PM, the vast majority are focal in origin and thus, non-reentrant. This mechanism is compatible with other findings, such as inducibility with isoproterenol or epinephrine infusion and burst pacing, and the finding that the first beat of tachycardia is typically similar to subsequent beats.19 In individual cases, Purkinje potentials are observed at the beginning of the QRS, suggesting the more superficial location of the focus. Some studies even suggested that the presence of Purkinje potentials at the site of origin and smaller size of the PM are associated with successful ablation of PM arrhythmias.20
Clinically, PM arrhythmias can exhibit mild spontaneous changes in QRS morphology. This can be explained by the location of the source closer to the tip of the PM and multiple exits towards the base. Indeed, Yamada et al. found spontaneous subtle changes in the QRS during an electrophysiology study, and were able to abolish PVCs with a single wide ablation at the base of the PM.13 This supports the hypothesis that variation of QRS is caused by multiple exits from a single intrapapillary focus. Furthermore, this finding can also be explained by the presence of other endocavitary structures that can connect the PMs, such as the chordae tendineae and false tendons.
Management
Patients with focal VT and or PVC originating from PMs generally have a good prognosis. Treatment is indicated mainly in symptomatic cases or when frequent ventricular ectopy (generally defined as >10–20% of all beats in a 24-hour recording) results in arrhythmia-mediated cardiomyopathy. The rare exemption is the scenario when PM houses the focal trigger for VF.7,8 Another situation is when PM is involved in reentrant arrhythmias in patients after myocardial infarction.4,5 In such cases, an ICD is generally recommended.
Catheter Ablation
Catheter ablation has emerged as the preferred treatment modality with the potential for complete cure, especially in patients without significant structural heart disease. When PVCs are frequent and/or VT is easily inducible and tolerated, exact identification of the source by activation mapping could be relatively easy. However, to make the mapping process systematic, online imaging is preferable. In our experience, ICE is an excellent tool. It allows assessment of catheter–tissue contact and optimal alignment of the catheter with the PM axis. In addition, ICE identifies focal scars within the PM that may correspond to the site of arrhythmia origin. For proper imaging of LV PMs, the ICE catheter is deflected across the tricuspid valve into the RV and rotated clockwise to achieve a long axis view of the LV with the posteromedial PM displayed first at the bottom of the LV, and anterolateral PM to the right upon further rotation of the catheter. For RV PMs, the imaging is easier, since the ICE catheter can be placed in close vicinity of the PMs.
Integrated ICE-guided imaging with electroanatomical mapping is another alternative that was demonstrated to have a higher success rate.21 Others use ICE with the magnetic sensor to enable an echo-facilitated 3D electroanatomical mapping and thus, real-time creation of precise geometries of cardiac chambers and endocavitary structures.22 Other less precise options include image integration of electroanatomical mapping with CT or cardiac magnetic resonance datasets.23
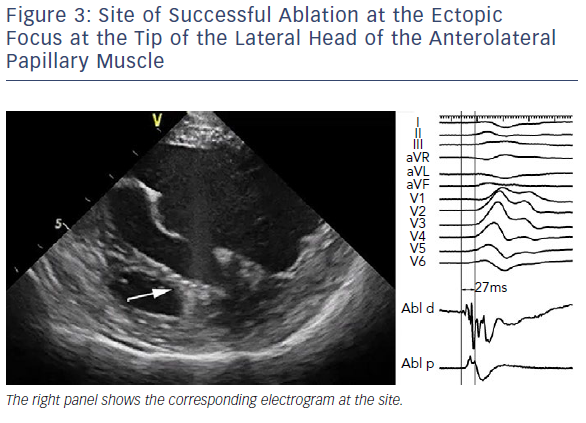
As aforementioned, catheter ablation of PM arrhythmias is typically performed at the site of the earliest endocardial activation identified by activation mapping. For this purpose, a multielectrode mapping catheter could be used in an attempt to achieve faster and more precise delineation of the site of origin of arrhythmia. Sites with activation preceding approximately −25 to −30 ms the QRS complex during ectopy suggest a close proximity to the arrhythmia focus (Figure 3). In some cases, a high-frequency Purkinje potential may be present at the successful ablation site. Pace mapping could be necessary when PVCs are infrequent. However, its utility is limited due to frequent catheter instability, and also due to the fact that it identifies only the exit site and not necessarily the source.
An earlier series by Yamada et al. showed that ablation at the site with excellent pace mapping frequently changed the ECG morphology, suggesting the source location to be higher or deeper along the muscle.13 A similar observation was described using an automatic pace mapping module to identify potential exits. In that study, morphological alterations were noticed in 92% of patients, requiring ablation at different exit sites.24 This suggests that the source is in the middle or apical portion of the PM, and pace mapping around the base of the PM identifies variable exits. Some groups advocated circumferential ablation around the base of the PM as a successful strategy.25
We recently confirmed that ectopic foci were located within the distal, mid, or proximal (basal) third of the PM in 67%, 19% and 14% of patients, respectively.10 A total of 86% of PM foci were acutely abolished, and very good long-term success was achieved in 65% of patients. Retrograde access into the LV was the most frequent approach (62%); however, a solely transseptal approach was selected in 29% of cases. Both approaches were utilised in 9% of cases. The location of arrhythmia focus at the tip of the PM, as determined by ICE guidance, was also described by others.26
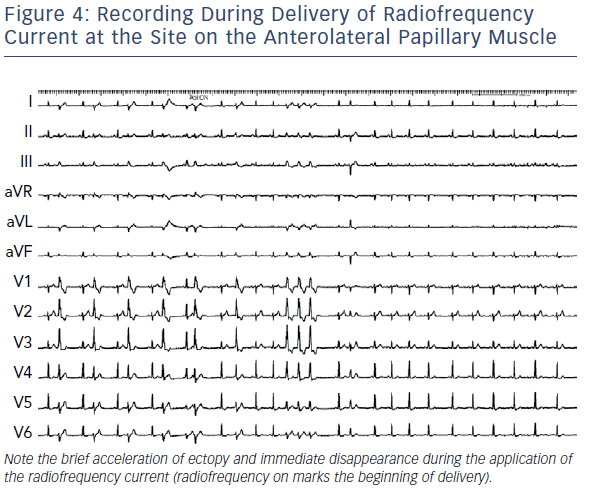
Interestingly, acceleration of the ectopy was observed during ablation on the PM in our series in 89% of patients (Figure 4).10 In two patients (6%), accelerated ventricular rhythm triggered VF, which required immediate defibrillation. Triggering VF during ablation at the PM was also reported by other groups.27
Regarding the set up for the ablation, we use the irrigated-tip catheter in the power-control mode starting at 30 W with an irrigation flow rate of 30 ml/minute for 30–60 seconds. We can titrate power to as high as 40 W, with the goal to achieve a decrease of 8–10 in the impedance and with a temperature limit of <40°C. The endpoint of the catheter ablation is the elimination and non-inducibility of VT or PVCs during an isoproterenol intravenous bolus and burst pacing from the RV (to a cycle length as short as 300 ms).
The main challenge for catheter ablation of PM foci is the catheter tip making contact with the tissue. Depending on the individual anatomy and rotation of the heart, retrograde or transseptal access could be preferable. More recently, some groups suggested the use of cryoablation to overcome problems with catheter instability. Comparing the efficacy of 8 mm cryoablation catheter versus 4 mm irrigated tip catheter showed 100% success in eliminating focus on the PM for cryoablation (12 patients), as compared with 78% for radiofrequency ablation (nine patients).28 In addition, no recurrences were observed in patients after cryoablation, while there was a 44% recurrence rate after radiofrequency ablation. The group at the University of Pennsylvania published their recent experience with cryoablation after a failure of radiofrequency ablation.29 They reported success in >90% of cases. In contrast, cryoablation has some disadvantages, such as less manoeuvrability, reduced lesion depth, and the inability to accurately track the catheter tip in the electroanatomical map. They reported on the use of a 6 mm Freezor Xtra catheter (Medtronic), which is able to fit through an Agilis or SL0 sheath.29 An 8 mm cryocatheter is considered less useful, as it is more difficult to manipulate, requires a larger diameter of the sheath, and may have limited reach due to its shorter length (90 cm).
Complications
In our experience, procedure-related complications are infrequent. When a retrograde aortic approach is used, damage to the aortic valve is a possibility. A few case reports have described worsening mitral regurgitation following ablation, and mechanisms may include PM dysfunction or catheter entrapment in the MV apparatus.30,31 VF induction may occur during radiofrequency application at the moderator band or PMs, requiring external defibrillation, and a new right bundle branch block may develop postprocedure in 40% of patients with moderator band ventricular arrhythmias.
Conclusion
The PMs are a recognised site of origin of ventricular arrhythmias both in patients without and with structural heart disease. The mechanism is predominantly focal and certain ECG features could be characteristic. Catheter ablation of focal sources is highly effective, but challenging. The reason is tremendous variability of the anatomy of PMs and instability of the ablation catheter on the moving structure. The use of ICE is fundamental to allow real-time visualisation of PMs, and ensure proper positioning of the catheter and tip-tissue contact. Cryoablation could be considered as an option to increase catheter stability and improve outcomes, especially in cases of failed radiofrequency ablation.
Clinical Perspective
- Ventricular arrhythmias originating from the papillary muscles of the left or right ventricle are mostly focal in origin and have a characteristic ECG pattern.
- Ectopic foci are predominantly located within the apical part of the muscles.
- Intracardiac echocardiography provides a unique tool to confirm the exact location of the focus identified by activation mapping.
- Although radiofrequency ablation has great potential to cure these specific arrhythmias, cryoablation may be a better alternative for some challenging cases, mainly due to better catheter tip stability.