Severe aortic valve stenosis (AS) is chronic, progressive illness that confers significant morbidity and mortality. Once symptomatic, patients with severe AS will ultimately succumb to the disease if it is not promptly corrected.1 Historically, surgical aortic valve replacement (SAVR) served as the exclusive therapeutic option to correct this mechanical problem.2 However, in 2002, Alain Cribier performed the first-in-man percutaneous implantation of a bioprosthetic aortic valve.3 Since this pivotal moment in the field of structural heart disease, transcatheter aortic valve replacement (TAVR) has developed into a contemporary, effective treatment option for severe AS. Multiple large, randomized clinical trials have demonstrated the superiority of TAVR versus medical therapy in the extreme surgical risk patient population, as well as the comparability of TAVR versus SAVR in all but the lowest surgical risk population to date.4–9 Since 2002, the equipment, techniques, and performance of TAVR continue to transform at a rapid pace.10 Yet, considerable work remains to fully eliminate the known complications of percutaneous valve replacement, including paravalvular leak and complete heart block requiring permanent pacemaker implantation, and to clearly determine the durability of transcatheter heart valves (THV).11 Furthermore, clinicians continue to seek innovative methods to minimize peri-procedural sedation, reduce hospital length of stay, and optimize cost effectiveness.12,13 In this focused review, we summarize the evolution of balloon-expandable and self-expanding THVs and highlight innovative valve platforms presently under investigation. We provide a timeline and summary of the landmark clinical trials showing favorable clinical outcomes following TAVR, as well as the evidence supporting its use in extreme, high, and intermediate surgical risk subgroups. We also emphasize exciting developments in peri-procedural TAVR management and adjunctive technological advancements that continue to revolutionize the field.
Commercial and Investigational Transcatheter Heart Valves
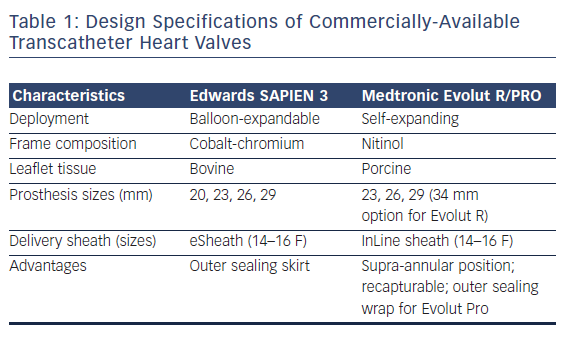
Two valve platforms are currently commercially available within the US – the Edwards SAPIEN balloon-expandable bioprosthesis and the Medtronic self-expanding bioprosthesis. The Edwards SAPIEN 3 THV is a trileaflet, bovine bioprosthesis based on a cobalt chromium frame, available in four sizes (20, 23, 26, and 29 mm) and deployed through a 14–16 French Commander Delivery System (transfemoral) or 18–21 French Certitude Sheath (transapical). With improvement in delivery sheath designs, the third-generation SAPIEN 3 THV can be advanced through 14–16 French expandable sheaths, whereas first-generation and second-generation THVs required much larger delivery profiles (up to 24 French and 20 French, respectively). In addition, as opposed to the older generation valves, the SAPIEN 3 THV comes with a polyethylene terephthalate skirt designed to reduce paravalvular regurgitation (PVR). Of historical note, the Edwards SAPIEN THV platform directly evolved from Dr. Alain Cribier’s initial design.
The Medtronic self-expanding, supra-annular valve consists of porcine pericardial tissue mounted on a nitinol stent frame. While the first-generation CoreValve® offered sizes between 26 and 31 mm delivered through an 18 French sheath, there is a wider breadth of sizing options with the newer generation Evolut™ PRO (23, 26, and 29 mm) and Evolut R (34 mm) bioprostheses. The Evolut PRO line, also a third-generation device, is delivered through a 16 French in-line sheath, thus allowing for a minimal luminal diameter of 5.5 mm for transarterial access. Routes for valve delivery include transfemoral, transaxillary, and transaortic access. A primary advantage of the new-generation Medtronic self-expanding valves includes the opportunity for recapturing and repositioning prior to permanent valve deployment. Furthermore, the Evolut PRO possesses an outer porcine pericardial tissue wrap to enhance prosthesis-to-annular contact and thus minimize paravalvular leak.
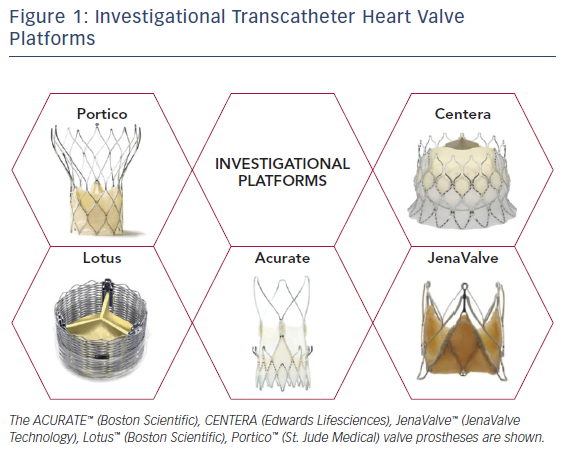
In complement to the evolving designs of the Edwards SAPIEN and Medtronic CoreValve THVs, there has been considerable effort devoted to the development and study of alternative percutaneous valve designs. Table 1 depicts design specifications of the newest generation, commercially available THVs approved for TAVR, while Figure 1 provides an illustration of investigational THVs. Each investigational THV is engineered with features designed specifically to address known challenges of valve deployment or complications following TAVR.
For example, the Lotus™ Valve System (Boston Scientific) is a mechanically expanded THV that consists of bovine pericardial tissue mounted on a woven nitinol stent frame. An outer adaptive seal is included on the valve specifically to minimize paravalvular leak, while the delivery system also permits retrievability and repositioning as needed. In addition, larger stent cell design purportedly may reduce the risk of coronary obstruction.14 Similarly, the Portico™ Valve (St. Jude Medical) is a self-expanding valve with a porcine pericardial sealing cuff. This valve is entirely resheathable prior to deployment (as opposed to Medtronic Evolut THV, which is partially recapturable). The valve leaflets and cuff are also treated with anticalcification technology.15 The CENTERA Valve (Edwards Lifesciences) is a self-expanding bovine valve that sits at the annular level on a nitinol stent frame. One unique feature of this valve’s delivery system includes a motorized, detachable handle.16 The JenaValve™ is yet another bioprosthesis composed of porcine pericardial tissue on a self-expanding nitinol stent, with CE-mark approval for transapical deployment.17 This THV possesses an active anchoring mechanism that resists valve migration during deployment.
TAVR Outcome Data for Extreme, High, and Intermediate Surgical Risk Groups
PARTNER-1 and PARTNER-2 Clinical Trials
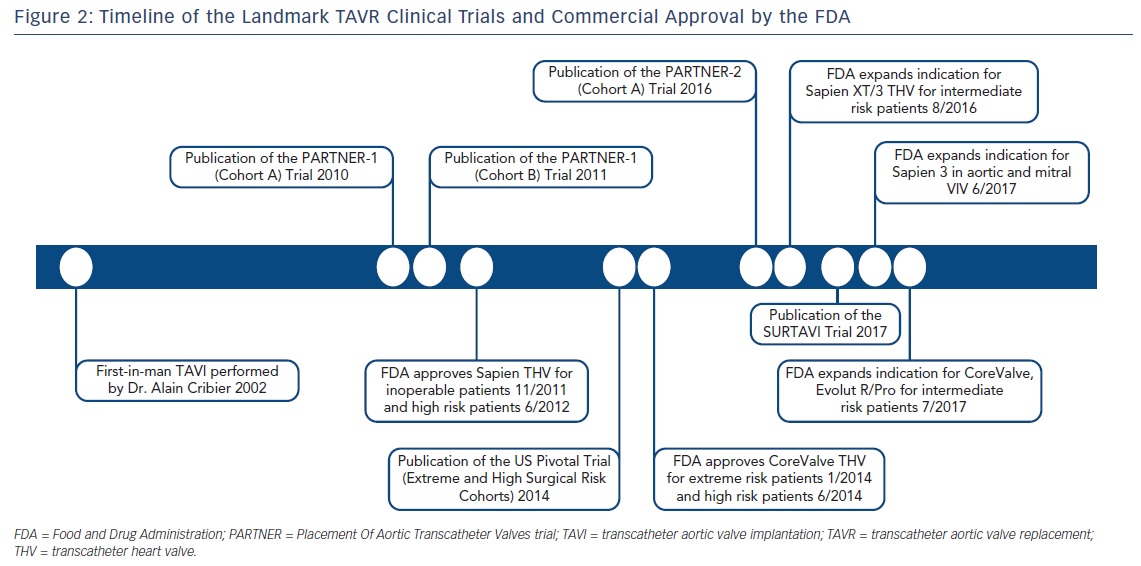
The 2- and 5-year data from the Placement Of Aortic Transcatheter Valves (PARTNER)-1 inoperable and high surgical risk cohorts were published in 2012 and 2015 (Figure 2).7,8,18,19 For inoperable patients (cohort B), mortality rates in the TAVR group using the first-generation, balloon-expandable, Edwards SAPIEN valve were 43.3 % compared with 68.0 % with standard therapy (p<0.001) at 2 years and 71.8 % versus 93.6 % (p<0.0001) at 5 years. Patients undergoing transcatheter valve therapy also exhibited substantially improved New York Heart Association (NYHA) Class symptoms. At 2 years, stroke rates were higher in the TAVR population (13.8 % versus 5.5 %; p<0.01), a difference driven predominately by early peri-procedural embolic events. However, at 5 years, stroke rates were comparable between groups (16 % versus 18.2 %; p=0.56). Finally, echocardiographic results adjudicated at an independent imaging core laboratory revealed durable prosthetic valve hemodynamics out to 5 years post-TAVR, and there was no evidence of structural deterioration of implanted THVs.
Meanwhile, in the high-risk PARTNER A cohort, all-cause mortality rates were not statistically different between the transcatheter and surgical valve replacement groups at 1 year (24.3 % versus 26.8 %; p=0.45), 2 years (33.9 % versus 35 %; p=0.78), and 5 years (67.8 % versus 62.4 %; p=0.76). Rates of hospital readmission, functional status, stroke or transient ischemic attack, and valve performance by echocardiography were all comparable between TAVR and SAVR. Of note, moderate or severe PVR occurred more frequently in the transcatheter group compared with surgery (6.9 % versus 0.9 %; p<0.001). Higher severity of PVR was also associated with increased late mortality post-TAVR (72.4 % versus 56.6 %; p=0.003 for severe versus mild/no aortic regurgitation). Overall, these longer-term data from PARTNER-1 supported the comparability of TAVR using a balloon-expandable THV versus SAVR in the high surgical risk patient population. Furthermore, for both the inoperable and high-risk PARTNER cohorts, THV hemodynamics remained stable out to 5 years without evidence of structural deterioration (inoperable: mean aortic valve area 1.52 cm2, mean gradient 10.9 mmHg; high-risk: mean aortic valve area 1.6 cm2, mean gradient 10.7 mmHg).7,8
In 2016, Leon and colleagues published results from the PARTNER-2A clinical trial, a study that randomized 2,032 intermediate surgical risk patients with severe AS to SAVR or TAVR (second-generation SAPIEN XT THV).9 At 2 years, all-cause death or stroke was similar between groups (19.3 % versus 21.1 %; p=0.25 for TAVR versus SAVR, respectively). While major vascular complications occurred more frequently in the transcatheter group than surgery (7.9 % versus 5.0 %; p=0.008, respectively) at 30 days, TAVR patients suffered lower rates of life-threatening bleeding (10.4 % versus 43.4 %; p<0.001), acute kidney injury (1.3 % versus 3.1 %; p=0.06), and new-onset AF (9.1 % versus 26.4 %; p<0.001). TAVR also compared favorably to SAVR in terms of intensive care unit (median 2 versus 4 days, p<0.001) and hospital length of stay (median 6 versus 9 days; p<0.001). Pacemaker implantation rates within 30 days (8.5 % versus 6.9 % for TAVR versus SAVR; p=0.17) and readmission rates at 2 years (19.6 % versus 17.3 %; p=0.22) were similar between groups. Notably, the frequency and severity of PVR were higher in the transcatheter group compared with surgery. TAVR patients with moderate or severe PVR suffered higher all-cause mortality than trace or no PVR (hazard ratio 2.85; 95 % CI [1.57–5.21]; p<0.001).
United States CoreValve Pivotal Trial and SURTAVI
In close succession to the PARTNER trials, the US Pivotal Trials studied the efficacy of the self-expanding Medtronic CoreValve for severe AS for the extreme and high surgical risk populations (Figure 2).4,5 The CoreValve Extreme Risk Pivotal Trial enrolled 489 patients in a non-randomized, single-arm study design. The primary endpoint of 12-month all-cause mortality or major stroke occurred in 127 patients (26 %). Individually, all-cause mortality occurred in 119 patients (24.3 %) and major stroke in 19 patients (4.3 %). There was a significant improvement in NYHA class symptomatology at 12 months post-valve implantation compared with baseline. In 2015, Yakubov et al published 2-year outcomes for this extreme risk cohort, showing an increase in the primary endpoint to 38.0 % (all-cause mortality 36.6 %, major stroke 5.1 %). Impressively, 94 % patients in the cohort reported NYHA class I or II symptoms. Valve hemodynamics remained durable at 2 years.20
The US Pivotal Trial for high surgical risk patients randomized 795 patients 1:1 to self-expanding CoreValve versus conventional surgical valve replacement. TAVR was superior to SAVR with respect to all-cause mortality at 1 year (intention-to-treat, absolute risk reduction 4.8 %; p=0.04 for superiority).4 At 3 years, the combined endpoint of all-cause mortality or stroke for TAVR versus SAVR was 37.3 % versus 46.7 %; p=0.006, respectively. There was an overall higher frequency of permanent pacemaker implantation following TAVR compared with SAVR present at 1, 2, and 3 years of follow-up (22.3 % versus 11.3 %, p<0.001; 25.8 % versus 12.8 %, p<0.001; and 28 % versus 14.5 %, p<0.001, respectively). However, TAVR performed favorably compared with SAVR with respect to major adverse cardiovascular or cerebrovascular events, all stroke, major or life-threatening bleeding, and acute kidney injury. Functional class by NYHA symptomatology was comparable between groups. Notably, TAVR patients treated through the ileofemoral access route experienced more rapid improvements in functional status and heart failure symptomatology compared with SAVR. The incidence of moderate or severe PVR was overall quite low in the study (6.8 %). Finally, mean aortic valve gradients at 3 years post-TAVR compared favorably to surgery, with no evidence of clinical valve thrombosis or structural deterioration in either group.6
Most recently, in 2017, Reardon and colleagues published results from the Safety And Efficacy Study Of The Medtronic Corevalve System In The Treatment Of Severe, Symptomatic Aortic Stenosis In Intermediate Risk Subjects Who Need Aortic Valve Replacement (SURTAVI) study.21 This intermediate surgical risk trial randomized 1,746 patients in 87 centers to TAVR using a self-expanding THV (CoreValve bioprosthesis 84 %, Evolut R 16 %) versus SAVR. At 24 months, TAVR was non-inferior to SAVR with respect to the composite primary endpoint of all-cause death or disabling stroke (12.6 % versus 14.0 %, respectively; 95 % credible interval [Bayesian analysis] for difference −5.2 to 2.3 %; posterior probability of non-inferiority >0.999). As seen in the high risk US pivotal trial, a higher proportion of patients undergoing transcatheter valve replacement suffered a major vascular complication (6.0 % versus 1.1 %) or required permanent pacemaker implantation (25.9 % versus 6.6 %). However, SAVR patients had higher rates of blood transfusions, acute kidney injury, and AF compared with TAVR. While the TAVR cohort demonstrated more favorable hemodynamic profiles on echocardiography compared with SAVR, moderate or severe PVR also occurred more frequently at 1 year with TAVR (5.3 % versus 0.6 %). The investigators concluded that TAVR was a non-inferior alternative to SAVR for patients with severe AS at intermediate surgical risk.
Minimalist TAVR
Traditionally, TAVR was performed under general anesthesia, deep sedation, endotracheal intubation with mechanical ventilation, and transesophageal echocardiographic guidance (TEE).12 However, in recent years there has been considerable interest in minimizing the amount of ancillary invasive procedures. Thus, experienced centers have successfully performed TAVR using local anesthesia and conscious sedation alone. In these cases, operators may elect to use transthoracic echocardiography or fluoroscopy alone for guiding valve deployment. This ‘minimalist’ philosophy is based on the theory that procedural time is reduced, recovery is expedited, and hospital length of stay can be shortened. Furthermore, the associated risks, albeit low, of general anesthesia, intubation, and TEE may be avoided completely. Opponents of minimalist TAVR cite the lack of randomized trial data to support the use of conscious sedation over general anesthesia or transesophageal echocardiographic versus transthoracic or fluoroscopic guidance during TAVR deployment. Furthermore, TEE is considered to be a very safe procedure with low event rates, even with conscious sedation.
Babaliaros and colleagues published results of minimalist versus standard approach TAVR based on data from their institutional experience.13 In this observational study, procedure room time, intensive care unit time, and hospital length of stay were all shorter for the minimalist approach. Meanwhile, 30-day clinical outcomes and survival beyond a median of 1 year were comparable between groups. The ensuing cost analysis performed also favored the minimalist approach versus standard care ($45,485 ± 14,397 versus $55,377 ± 22,587; p<0.001). Regardless, the minimalist approach should be determined on a case-by-case basis and vetted by the multidisciplinary heart team during the pre-procedural planning period.
Cerebral Embolic Protection
While stroke rates post-TAVR have improved over time, there is enthusiasm around the development of devices to further minimize this risk.22–24 For example, the Sentinel™ Cerebral Protection System (Claret Medical Inc.) recently received US Food and Drug Administration (FDA) clearance for use during TAVR. This device consists of a dual-filter system with a 6F articulating sheath delivered via the right radial artery. The proximal nitinol filter (15 mm) is situated in the right brachiocephalic artery while the distal filter (10 mm) is positioning in the left common carotid artery.
In the Sentinel clinical trial,25 although there were no statistically significant differences in terms of major adverse cardiac and cerebrovascular events or new lesion volume by MRI, investigators reported 99 % debris captured by the device post-TAVR. In a recent propensity-matched analysis published by Seeger and colleagues, use of cerebral protection with the Sentinel device was associated with a reduced rate of disabling and nondisabling stroke (OR 0.29; 95 % CI [0.10–0.93]; p=0.03) as well as reduced composite primary endpoint of all-cause mortality or all-stroke (OR 0.30; 95 % CI [0.12–0.77]; p=0.01). While this study was not randomized, it does suggest a potential neurological benefit of cerebral protection during TAVR.26
Another device – the TriGUARD™ 3 HDH Embolic Deflection Device (Keystone Heart) – uses a polymeric mesh that fully covers the supra-aortic trunk takeoffs, delivered through an 8F system from the groin. This device is currently under investigation and not commercially available for use in the US (the Cerebral Protection to Reduce Cerebral Embolic Lesions After Transcatheter Aortic Valve Implantation [REFLECT] trial is ongoing [ClinicalTrials.gov NCT 02536196]). Further study is merited to understand the specific immediate and long-term advantages that may be conferred by these innovative technologies.
Expanding Indications
Given the game-changing success of TAVR in the treatment of calcific aortic valve stenosis, tremendous efforts have ensued to apply this technology to other forms of valvular heart disease including: congenital bicuspid aortic valve stenosis; prosthetic valve degeneration; mixed or pure aortic regurgitation; mitral, tricuspid, or pulmonic valve diseases; and alternative AS patient populations (e.g. asymptomatic severe AS, moderate AS with left ventricular dysfunction).27 For instance, several institutions have published observational studies that proclaim the feasibility and safety of performing TAVR for bicuspid AS.28–30 However, there have been no large-scale, randomized control trials designed specifically for this congenital patient population. Lingering concerns regarding the percutaneous treatment of congenital bicuspid aortic valve disease include concomitant ascending aortic disease and dilatation, unusual valve geometries, and unknown long-term durability of THVs, particularly with respect to this inherently younger patient cohort. Several of these critiques are also echoed for transcatheter therapy in non-calcified valvular lesions such as mixed or pure aortic regurgitation, in which there may be higher risk for valve malapposition and embolization. Therefore, additional investigation – ideally in the context of randomized comparative data – is warranted prior to widespread expansion of TAVR to such populations.
In addition, transcatheter valve-in-valve (ViV) therapy has received growing attention as a means to avoid redo sternotomy for patients with failed surgical bioprostheses. The Valve-in-Valve International Data Registry introduced in 2010 represents the largest global experience of aortic ViV to date, and 30-day mortality was 7.6 % in this cohort.31,32 While aortic ViV is associated with lower frequencies of PVR and pacemaker implantation, there are higher reported rates of malpositioning, coronary obstruction, and residual prosthetic valve gradients. ViV procedures have most commonly employed the use of the Medtronic CoreValve and Edwards SAPIEN XT THV, although there have been published reports using alternative prostheses such as the Portico and SAPIEN 3 valves. Notably, in June 2017, the US FDA expanded the approval indication for the SAPIEN 3 THV in failed bioprosthetic valves in the aortic or mitral positions for high-risk patients.
Finally, one of the remaining patient populations of immense interest is the low surgical risk cohort. While the PARTNER-1, PARTNER-2A, US Pivotal, and SURTAVI clinical trials have substantiated the effectiveness of TAVR for extreme, high, and intermediate risk subgroups, current trials are underway to randomize low-risk patients to either surgical or percutaneous valve replacement. These include PARTNER-3 (The Safety and Effectiveness of the SAPIEN 3 Transcatheter Heart Valve in Low Risk Patients with Aortic Stenosis; ClinicalTrials.gov NCT02675114), and the Medtronic Transcatheter Aortic Valve Replacement in Low Risk Patients (ClinicalTrials.gov NCT02701283). In 2016 and 2017, respectively, the US FDA granted an expanded indication approval for the balloon-expandable, Edwards SAPIEN 3 and SAPIEN XT THVs and the self-expanding, CoreValve and Evolut R/Pro THVs in intermediate-risk patients with severe AS.
As the indications for TAVR have expanded over time (Figure 2), vigorous debate continues with respect to the lowest surgical risk patient population. For instance, the long-term durability of transcatheter bioprosthetic valves beyond 5 years is as yet unclear. In addition, implanting THVs in younger, low-risk patients may confer an increased downstream probability of requiring more ViV procedures over their collective lifetimes. With each additional ViV TAVR, a patient is subject to the risks of procedural complications, obstructed coronary access over time, increased residual THV gradients, and reduced aortic valve areas for body size (i.e. patient–prosthesis mismatch). Furthermore, the lifetime impact of residual paravalvular leak or permanent pacemaker in this patient group is of concern and should be prospectively studied. Data from the low-risk Edwards and Medtronic clinical trials will hopefully clarify the clinical impact of the above concerns, including THV durability several years post-implantation (i.e. 10 years or more), and of course, inform us of the comparability of TAVR and SAVR in this lowest-risk subgroup.
Conclusions
Since Alain Cribier performed the first-in-human TAVR in 2002, we have witnessed an unparalleled revolution in the field of structural heart disease and intervention. Data from the PARTNER-1 and US Pivotal Trials support the efficacy of TAVR out to 5 and 3 years, respectively, although the longer-term durability of THVs beyond this time frame remains to be seen. Recently, the PARTNER-2A and SURTAVI clinical trials have consistently demonstrated the non-inferiority of TAVR to SAVR in intermediate-risk patient populations, while we anticipate results from the low-risk clinical trials (e.g. PARTNER-3), presently in enrolment. In the past decade, the designs of the traditional balloon-expandable and self-expanding THVs have steadily evolved in attempts to attenuate known risks of TAVR, such as paravalvular leak and pacemaker implantation. Examples of design modifications incorporated into newer device iterations include sealing skirts and re-sheathable platforms. Furthermore, there has been a movement to make TAVR as minimally invasive as possible, with some operators performing this procedure under local anesthesia, conscious sedation, and fluoroscopic guidance alone. At the heart of the TAVR movement, the consistent foundation for success throughout this era is the multidisciplinary heart team approach to patient care. By leveraging the skills and expertise of interventional cardiologists, cardiac surgeons, anesthesiologists, nursing, and other allied health professionals, the opportunity to provide patient-centered, holistic care is achieved for any patient being considered for surgical or percutaneous aortic valve replacement.