Diffuse Coronary Stenosis
A diffuse coronary stenosis is customarily defined as any stenosis exceeding 20 mm in length in the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions (ACCF/AHA/SCAI) guidelines for PCI.6 This cut-off value has not been used in trials of diffuse stenosis, which have rather used the term ‘long lesion’ with no single cut-off being used consistently. Diffuse stenoses have been associated with an increased risk of restenosis with a 17 % increased risk of major adverse cardiac events (MACE) and a 20 % increased risk of target lesion revascularisation (TLR) for every 10 mm of first-generation drug-eluting stents (DES) deployed.1,7 Higher rates of stent thrombosis, death and MI at 3 years with DES lengths higher than 31.5 mm compared with shorter stents have been reported.2 Retrospective IVUS analysis have shown higher TLR rates with stents longer than 40 mm,8 while registry data have shown a 58 % increased risk of very late stent thrombosis with >28 mm of first-generation DES3 0.3 %; 1 year, 0.6 %; and 5 years, 1.6 %. The increased rates of in-stent restenosis, stent thrombosis and late-stent thrombosis led to the hypothesis that intravascular imaging in this subset of complex lesions may be beneficial.
Intravascular Ultrasound Studies of Long Lesions
An advantage of IVUS guidance in diffuse stenosis was demonstrated by the Thrombocyte Activity Evaluation and Effects of Ultrasound Guidance in Long Intracoronary Stent Placement (TULIP) study in the bare-metal stent (BMS) era and also by a single-centre observational study in the DES era.9 However, the applicability of these results were questioned, especially following the negative results with IVUS-guided management of long lesions in the Real Safety and Efficacy of a 3-Month Dual Antiplatelet Therapy Following Zotarolimus-Eluting Stents Implantation (RESET) study.10 The interest in IVUS in diffuse stenosis, however, has been rekindled following the publication of the Impact of Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions (IVUS-XPL) study, which demonstrated a benefit with IVUS guidance.11
The TULIP study, a randomised controlled trial of IVUS versus angiographic-guided implantation of BMS in long lesions, demonstrated favourable angiographic and clinical outcomes in patients with long coronary lesions (>20 mm) treated with BMS (>3 mm) under IVUS guidance.12 The TULIP study criteria for optimisation were achieved in 89 % of patients. IVUS guidance led to a reduction in rates of binary restenosis (23 % versus 43 %; P=0.08) and TLR (4 % versus 14 %; P=0.037).12 Despite the benefits of IVUS in long lesions shown in the TULIP trial, with the advent of DES, the applicability of the results of BMS IVUS studies to DES has started being questioned.
In the DES era, a single-centre observational study of IVUS versus angiography-guided PCI in diffuse coronary artery disease indicated that IVUS guidance significantly decreased rates of MACE and TLR at 2 years.9 In this cohort further adjuvant balloon dilation was performed whenever stent underexpansion, defined as <5 mm2 of minimum stent area, or malapposition with IVUS were present.
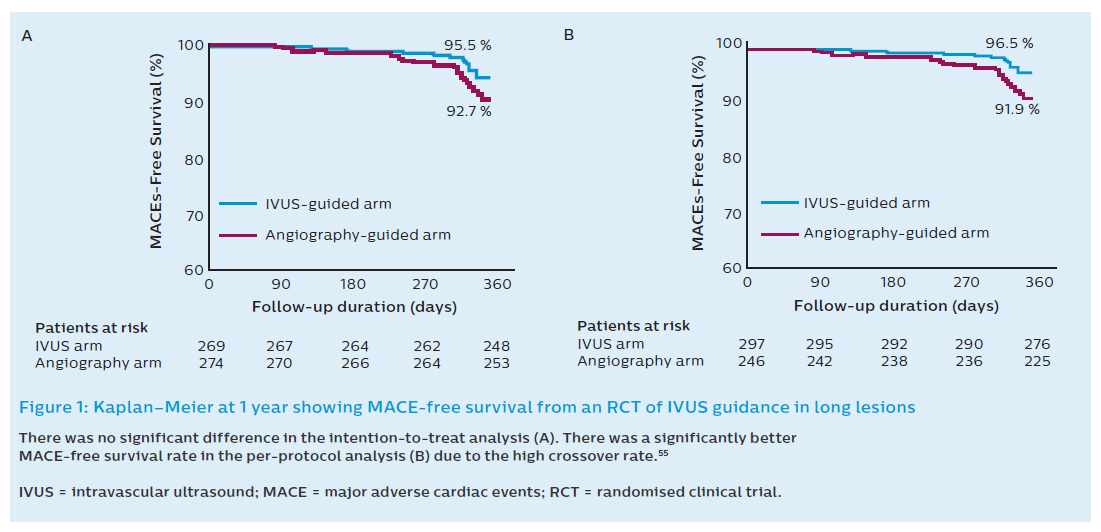
The first randomised IVUS guidance study of long lesions in DES was a substudy of the RESET trial.10,13 RESET was a randomised, open-label, multicentre trial designed to demonstrate the non-inferiority of 3 months of dual antiplatelet therapy (DAPT) following Endeavor® Sprint zotarolimus-eluting stent implantation compared with 12 months of DAPT with another DES. Subjects were subsequently randomised to ultrasound or angiographic guidance in a 2x2 design. The study protocol did not specify the adjunctive measures to be taken based on IVUS findings and did not specify IVUS criteria of optimal stent expansion. Furthermore, pre-intervention IVUS was only performed in 53 % of the IVUS-guided arm. Chronic total occlusions (CTOs) and two-stent approach bifurcation lesions were excluded. The primary endpoint was MACE (cardiovascular death, MI, stent thrombosis or target vessel revascularisation) at 12 months. The total stent length was approximately 32 mm in both arms. In the intention-to-treat analysis MACE occurred in 12 patients (4.5 %) in the IVUS-guided arm (n=297) and 20 patients (7.3 %) in the angiography-guided arm (n=297; P=0.16). Although this was a negative trial, using the intention-to-treat analysis there was a significant number of crossover. IVUS guidance was not used in 13 patients (4.8 %) in the IVUS group while 20 patients (7.3 %) in the angiography-guided group had IVUS performed due to the operator’s decision. When the use of IVUS was analysed as per operator decision (per protocol) the MACE rates were significantly lower in the IVUS-guided arm (4.0 %) when compared with the angiography-guided group (8.1 %; P=0.048; Figure 1). This study was inadequately powered due to the lower-than-predicted event rate and the high crossover rate. Furthermore, the operators in the angiography-guided group were also experienced in IVUS use and this may lead to different PCI approaches compared with non-IVUS users.10
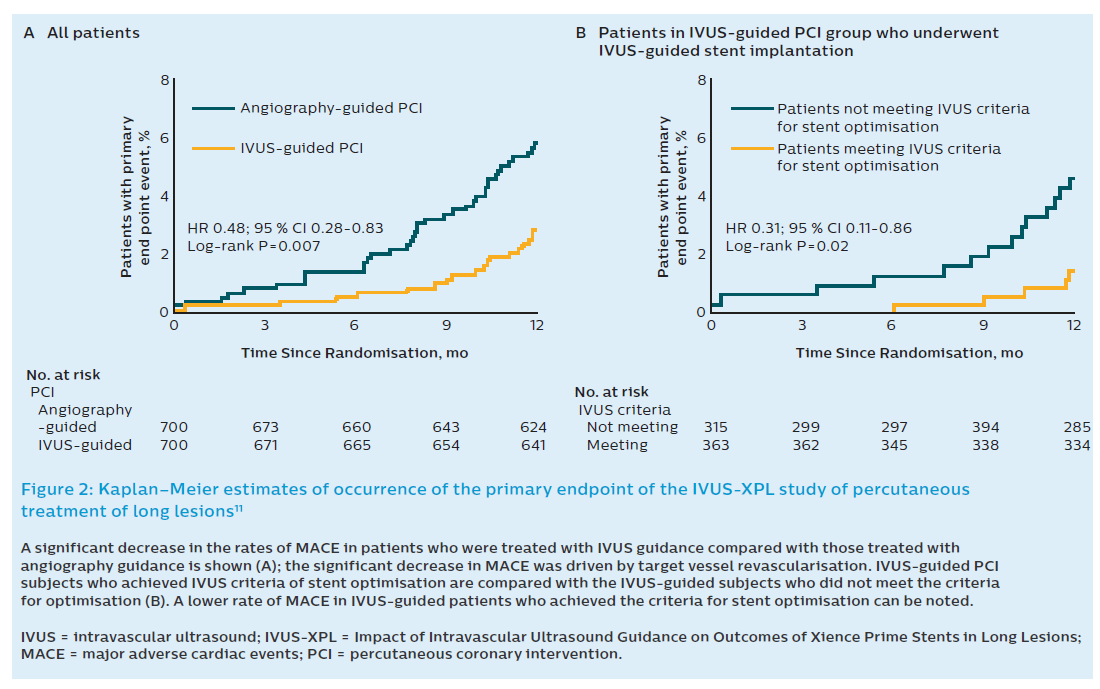
The second randomised clinical trial (RCT) studying IVUS guidance in long lesions was the IVUS-XPL study.11 The IVUS-XPL study randomised 1400 patients across 20 Korean centres to IVUS-guided implantation or angiography-guided implantation. The mean stented length was 39.3 mm and only Xience prime stents were used. An angiographic residual diameter stenosis of <30 % by visual estimation and the absence of angiographic dissection was required in the angiographic group. IVUS measurements were used to choose stent size and length. At 1 year, 19 patients (2.9 %) in the IVUS-guided group had a MACE, while in the angiography-guided group, 39 (5.8 %) had a MACE (hazard ratio [HR] 0.48; 95 % CI 0.28–0.83; P=0.07). This equates to a 48 % relative reduction in risk when using IVUS guidance for long lesions. This was driven by a reduction in the rate of ischaemia-driven TLR in the IVUS-guided cohort (2.5 % versus 5 %; HR 0.51; 95 % CI 0.28–0.91; P=0.02). Cardiac death and target lesion-related MI did not differ between the groups. As expected, use of adjunct poststent balloon dilation was more common in the IVUS group than the angiography group (76 % versus 57 %; P<0.001; Figure 2). A greater mean final balloon size as well as postinterventional minimum lumen diameter and area were achieved with IVUS, particularly in the 54 % of patients who met the IVUS criteria for optimisation of stent placement. The IVUS criteria for stent optimisation in this study was defined as a minimal lumen cross-sectional area greater than the lumen cross-sectional area at the distal reference segments. This criterion was achieved in 54 % of subjects. In the latter, there was a lower event rate (1.5 % versus 4.6 %; HR 0.31; 95 % CI 0.11–0.86; Figure 2B). Interestingly, there was no difference in adjunct postdilatation, final balloon sizes and maximal inflation pressures between those who achieved IVUS-defined optimal stent implantation and who who did not.11
The positive results of the IVUS-XPL study are all the more impressive when the low overall event rate is kept in mind. However, despite the positive nature of this study, the applicability of its results are limited by the lack of clear criteria for stent choice and optimisation. The clinical benefit of IVUS-guided DES implantation may be attributed to the larger minimal lumen diameter followed by the more frequent adjunct postdilatation with a large-sized balloon in the IVUS-guided group.
Overall, findings from these two RCTs indicate that IVUS guidance in the treatment of long lesions may improve outcomes in the DES era. Events seem to be especially low achieving a minimum stent area greater than the target reference diameter; however, this was only possible in just over half of the cohort, showing how difficult the management of these lesions can be.
Intravascular Ultrasound Guidance in the Management of Diffuse Lesions
The RCTs mentioned above do not provide clear criteria for the use of IVUS. The following sections will attempt to fill this void by discussing important aspects of IVUS use that are particularly important in the management of diffuse lesions.
Pre-percutaneous Coronary Intervention Intravascular Ultrasound Assessment
An important aspect of PCI in long stenoses or diffusely diseased segments is the selection of appropriately sized coronary stents. Coronary tapering occurring as a result of branching occurs frequently over long coronary segments; at the time of stent selection this has to be differentiated from the luminal tapering due to atherosclerosis. Achieving maximal luminal dimensions post stenting relies on safe deployment of larger stents, and assessing the extent of positive remodelling may facilitate this. Alternatively, the presence of negative remodelling may trigger a more conservative attitude for the sake of safety.
Many major IVUS studies in the DES era have concentrated solely on the role of IVUS for stent optimisation and have not detailed how pre-PCI IVUS should be used for stent selection. Overall, in practice three questions can be answered by IVUS when selecting stent dimensions: 1) What are the luminal dimensions at the proximal and distal landing zones? 2) What are the external elastic membrane (EEM) dimensions along the segment to be treated, and at which points do the EEM dimensions change significantly? 3) What is the length between the proximal and distal landing zone?
By principle, the deployment of the stent in the landing zones should entail the minimal risk of coronary dissection. This justifies the use of the distal luminal diameter, and not of EEM, to select stent size especially in the presence of significant atheroma. In a long lesion, the distal and proximal landing zone dimensions are rarely the same and in most cases a significant increase in calibre can be expected proximally. To obtain the largest stent area possible, postdilatation can be performed with non-compliant balloons sized to the proximal luminal diameter; however, making sure that it does not exceed the EEM diameter across the segment to be postdilated. Special consideration to the EEM diameter along the stenotic segment must be made, especially in the case of negatively remodelled stenoses, to avoid the risk of vessel rupture.
The selection of stent diameter will also have to take into account the limits of stent expansion. When stenting across large branches there may be a significant difference between the distal reference lumen diameter and the proximal reference lumen diameter. The stent selected according to the distal reference diameter must be capable of being postdilated up to the proximal reference diameter without damage to its structure.14
Another important consideration besides stent diameter is stent length. Besides covering the selected stenosis, choosing the best landing zone possible is of utmost importance to decrease the risk of edge dissections. IVUS is superior to angiography in determining appropriate reference segments to act as landing zones for stent deployment. This is due to the fact that IVUS often reveals a significant plaque burden, even in segments that appear normal on angiography. Reference segments chosen angiographically that are often assumed to be free of plaque have an average plaque burden of 30–50 %.15 A key aspect of IVUS guidance in long stenoses is the identification of landing zones that allow selection of the shortest stent length possible while reducing to a minimum the risk of edge dissections.
Some authors have defined a reference coronary segment in IVUS as that with a cross-sectional image adjacent to the stenosis with <40 % plaque burden.16 Edge restenosis is mostly observed in cases of residual plaque area >50 % at the stent margins. Stepwise IVUS criteria for landing zone selection state that ideally normal-appearing lumen should be chosen, but when this is not possible, a site with <50 % plaque burden or alternatively a site with the least-possible plaque burden along the artery have been proposed. Using these criteria, improved rates of stent restenosis and TLR at 8 months’ follow-up in 162 consecutive patients with 180 lesions treated with sirolimus-eluting stents (SES) has been demonstrated.17 Yet, in practice, a trade-off between locating an atheroma-free landing segment and reducing as much as possible DES length has to be made.
Role of Intravascular Ultrasound Co-registration
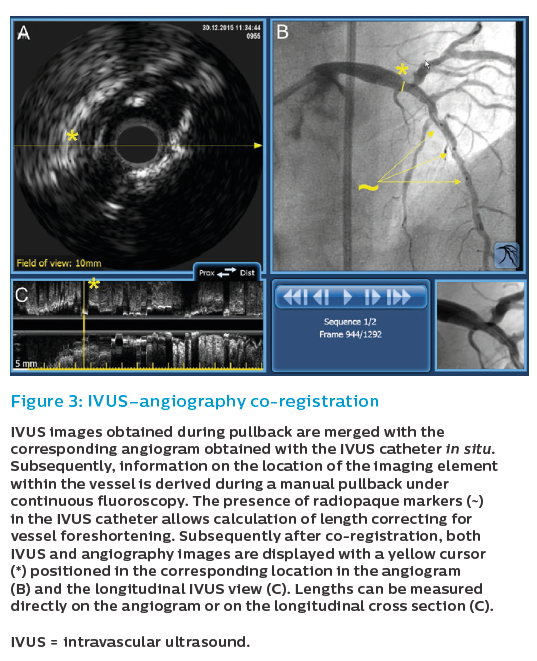
Both diagnostic and procedural guidance use of IVUS face the challenge of translating intracoronary imaging findings to specific locations in the angiogram. This may lead to significant geographic mismatches in IVUS-guided PCI. The Does Optical Coherence Tomography Optimize Revascularization (DOCTOR) study clearly showed that even experienced interventional cardiologists were unable to fully integrate intravascular imaging findings made using standalone systems.18 In this study 70 % of target lesions selected using Optimal Cohorence Tomography (OCT) were left uncovered and the position of the deployed stent varied by 5.4±2.6 mm compared with the landing zone chosen on OCT. Although this was an OCT study, the issues are identical as with IVUS systems.
These issues are addressed by IVUS–angiography co-registration (Figure 3). The co-registration system merges in real time the information obtained with intracoronary imaging and angiography, allowing the operator to easily see where each IVUS frame’s position is on the angiogram. Using co-registration, the operator has the confidence that the actions triggered by IVUS findings are accurately performed in the chosen segment. Such actions may include the selection of stent landing zones, safe use of larger balloons for post-dilation in areas within long stenoses with large EEM areas, or high-pressure post-dilation in underexpanded stent segments.
Role of Coronary Physiology in the Management of Diffuse Stenoses
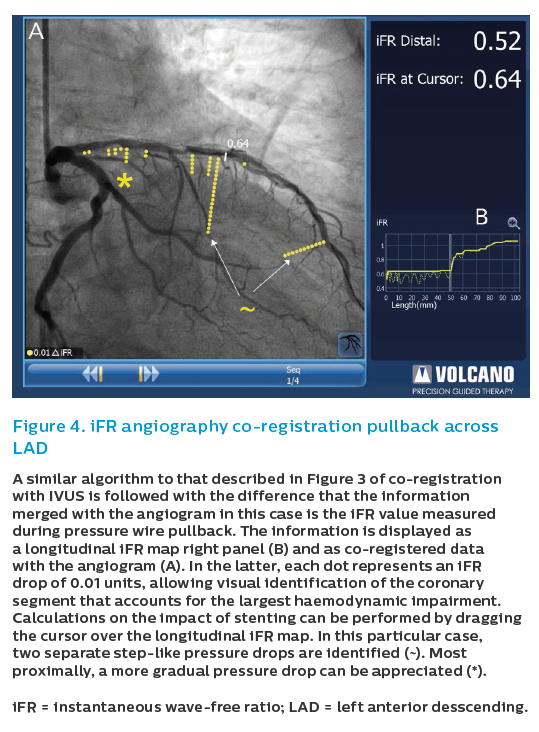
In addition to its key role in assessing stenosis severity, over the last years a growing interest in the use of physiology for PCI guidance has emerged. In the context of long diffused coronary segments, longitudinal pressure mapping often reveals specific locations that concentrate most of the haemodynamic impairment, and this may have implications for procedural PCI decision. This type of longitudinal vessel mapping can be currently performed with great ease, particularly by obviating the use of intravenous adenosine infusion by using instantaneous wave-free ratio (iFR), and by the facilitated analysis performed by dedicated consoles (Figure 4). By identifying where the greatest drop in iFR occurs across the coronary artery during an iFR pullback, the operator can gain insights into which segments need to be treated to gain the best haemodynamic result. These insights help the operator select segments for stenting that will yield the highest haemodynamic gain and exclude others that are not causing haemodynamic compromise.19
This information can be integrated with intracoronary imaging findings. Not seldom, the segment identified with physiological mapping does not coincide exactly with the optimal landing zones for stenting. IVUS can be used to fine-tune stent length in order to address the physiological findings while ensuring that stent deployment is performed optimally. Recently, angiographic co-registration with iFR and IVUS (tri-registration) has been launched to facilitate these aspects.
Post-percutaneous Coronary Intervention Assessment and Optimisation
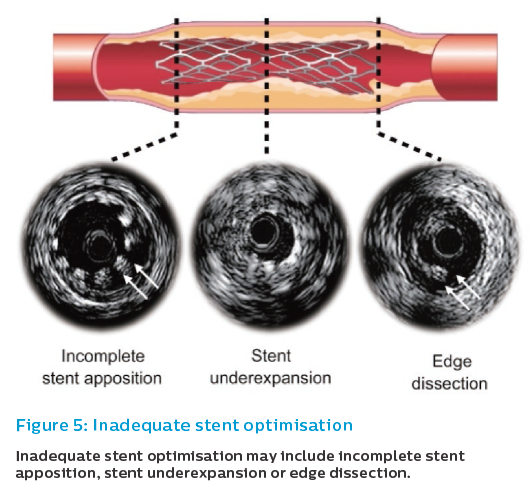
Overall, post-implantation IVUS guidance aims to minimise the occurrence of IVUS-related predictors of adverse events after PCI. IVUS-based predictors of outcome after PCI include smaller minimal stent area (MSA), stent underexpansion, stent edge dissection, incomplete stent apposition (ISA), and incomplete lesion coverage (Figure 5).8,20–26
Suboptimal stent deployment conditions have been reported to be potent IVUS predictors of stent restenosis and stent thrombosis,24,27,28 suggesting that stent implantation under IVUS guidance still has a role in the DES era. IVUS allows more aggressive intervention using a larger diameter balloon while avoiding the risk of vessel perforation and side branch occlusion.29,30 This evidence is based on studies including all type of coronary stenosis, without specific studies dedicated to long coronary stenosis.
Stent Underexpansion
Stent underexpansion refers to areas of a stent that have a decreased MSA compared with a reference segment or an expected area based on balloon area.31 The clinical relevance of this phenomenon is that it has been clearly identified with stent failure, both restenosis and thrombosis.8,22–25
The potential of IVUS in addressing this problem is that stent underexpansion frequently occurs despite using a proper deployment technique. In an IVUS study of DES expansion only 75 % of deployed stents achieved the predicted minimal stent diameter and 66 % the predicted MSA despite high pressure balloon inflation and an adequate angiographic result.32 In the Stent Optimization (STOP) study that investigated routine and IVUS-guided postdilatation, adequate expansion was reached in 21 % after stent deployment, in 54 % after routine postdilatation and in 81 % after IVUS-guided postdilatation.33
Although there are no dedicated studies, there is a consensus that the possibility of stent underexpansion increases with stenosis length. Aggressive attempts using higher pressures and larger balloons are often not resorted to in angiographic-guided procedures due to the risk of vessel–balloon mismatch, arterial perforation and loss of side branches,30,34 and, in this regard, a more accurate assessment of vessel and stent dimensions may contribute to a safer optimisation, which may be selectively applied in focal areas of underexpansion within the implanted stent. An arbitrary MSA cut-off value of 5 mm2 is often recommended for use in non-left main lesions with DES based on the Sirius study, which used SES; this study was not dedicated to long lesions.35,36 In the IVUS-XPL study, an RCT of long lesions, optimal stent expansion was defined as a cross-sectional area (CSA) greater than the distal lumen reference segment.11 In a pooled analysis of 804 patients with long lesions from the IVUS-XPL and RESET studies treated with second-generation stents, two independent IVUS predictors of MACE were an absolute MLA of 5.0 mm2 and a relative enlargement of the MLA to the distal reference segment lumen area showed a high negative predictive value for adverse events. Achieving an MLA ≥5.0 mm2 is mechanically impossible at the distal portion of long lesions that land adjacent to a small-sized distal reference segment. In this subgroup of patients achieving an MLA at least the same as the distal reference segment lumen area was associated with better hard outcomes.37
Criteria for expansion are difficult to standardise across the wide range of scenarios. A single cut-off value of 5 mm2 cannot hold true across the wide range of coronaries and is often difficult to achieve in small vessels. Comparing the MSA to the distal reference, although more laborious, may give a better indication of good expansion across the whole range of vessel diameters. An MSA >90 % of the distal reference lumen was achieved in all patients in the HOME-DES trial, while the more ambitious target of MSA greater than the lumen CSA at the distal reference used in the IVUS-XPL study was only achieved in 54 %. In the latter study, there was a significant difference in the outcomes between those who achieved this target and those who did not. Even when this criterion is reached, in long lesions, due to the difference between distal and proximal vessel calibre, adequate stent expansion defined by a distal reference may not necessarily be an adequate definition for the more proximal segments.
Incomplete Stent Apposition
ISA refers to the presence of lumen on the outer border of stent struts (‘floating struts’) and has been associated with repeat revascularisation due to stent restenosis and stent thrombosis.38,39 Specific imaging algorithms that allow colour coding of coronary flow (e.g. ChromaFlo®) may assist in recognising ISA. Vessel/stent mismatch is the main cause of ISA rather than stent underexpansion.40 Adequate stent sizing by IVUS may be clinically important in preventing ISA and in optimising initial stent deployment. Besides immediate inadequate stent apposition due to vessel/stent mismatch, late-acquired inadequate stent apposition has been described possibly secondary to positive remodelling after stenting and disappearance of jailed thrombus in acute coronary syndromes.41 As with stent underexpansion, there is a generalised consensus that treatment of longer, diffuse stenoses with shifting vessel diameters in the stented segment may increase the possibility of focal areas of ISA.
Edge Dissection
Edge dissection, which is complicated by lumen narrowing <4 mm2 or dissection angle ≥60°, has been associated with an increased incidence of early stent thrombosis (ST).42 Minor dissections detected by IVUS may not be associated with an increased incidence of ST, therefore, although no consensus exists a conservative strategy may be possible.43,44 Edge dissection might be caused by stent oversizing at the landing zone, aggressive postdilation with large balloons, or high plaque burden at the landing zone. As discussed in different sections of this article, these aspects can be addressed with IVUS interrogation, with the potential of increasing procedural safety.
IVUS guidance equates to good apposition of stent struts, adequate stent expansion to obtain MSA greater than the distal reference lumen CSA,11 and lack of major dissections, intramural haematomas and geographic misses.45 As seen in major studies, achieving all these criteria is not always possible and, therefore, one must not get carried away seeking to tick all the boxes at the expense of decreased procedural safety.
Algorithm for Diffuse Stenoses
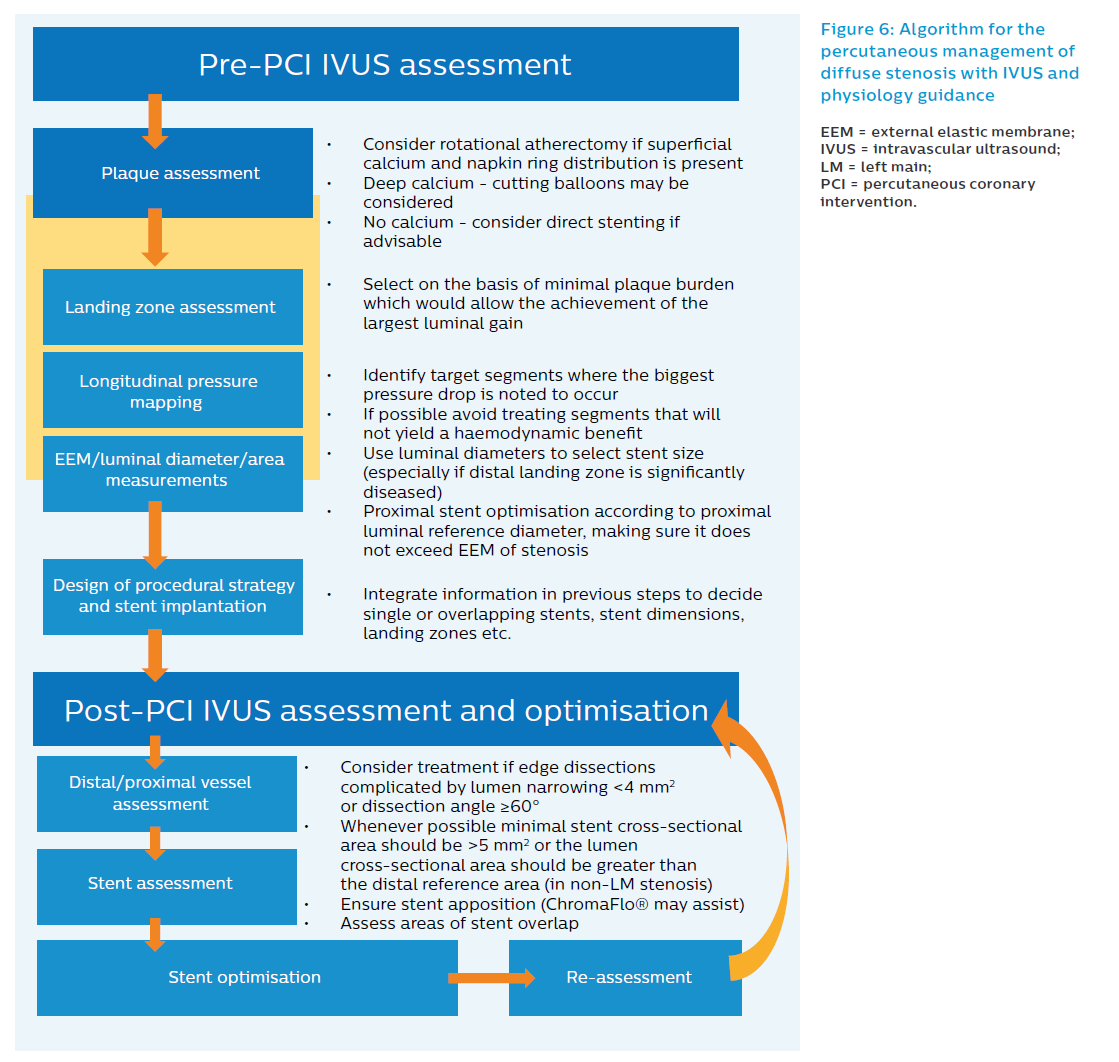
The presented algorithm for long lesions (Figure 6) takes into account all the factors discussed in the previous sections. The algorithm reflects the current clinical practice at Hospital Clinico San Carlos (Madrid, Spain), but has not been formally investigated and is therefore not a reflection of what is recommended in the current guidelines. The workflow algorithm can, however, be useful in helping to support research and clinical decision making.
- We recommend that IVUS be used both before and after stent implantation rather than just for stent optimisation.
- In the presence of three or more arcs of superficial calcium (versus deep calcium), lesion preparation with rotational atherectomy may be considered. In contrast, if the calcium appears deep, scoring balloons may be considered. In the absence of calcium, direct stenting may be considered whenever indicated.
- In the absence of co-registration during IVUS pullback, angiographic reference segments should be identified and correlated to IVUS frames of interest.
- Longitudinal pressure mapping provides information about which segments are causing the most haemodynamic compromise and can help predict what the haemodynamic gain of stenting a particular segment may be. This information often changes stenting strategy. The iFR longitudinal pressure mapping data and the choice of adequate landing zones must both be taken into consideration when selecting stent length and landing zone.
- Choosing optimal stent size:
- Stent diameter selected according to average distal reference luminal diameter ensuring that EEM area at the stenosis is not superseeded.
- Non-compliant balloon for proximal optimisation should be selected according to proximal luminal reference diameter, ensuring it does not exceed EEM of lesion at any point and avoiding edges if they are significantly diseased. Post-implantation IVUS definitions aim to confirm the following:
- Apposition: all struts apposed to vessel wall.
- Adequate expansion: MSA >5 mm2 or minimal stent cross-sectional area greater than the lumen CSA at distal reference segments (in locations other than left main).11
- Exclude edge dissection: treat if complicated by lumen narrowing <4 mm2 or dissection angle ≥60°.
- Exclude incomplete lesion coverage.
Special Considerations with Respect to Diffuse Stenoses
Chronic Total Occlusions
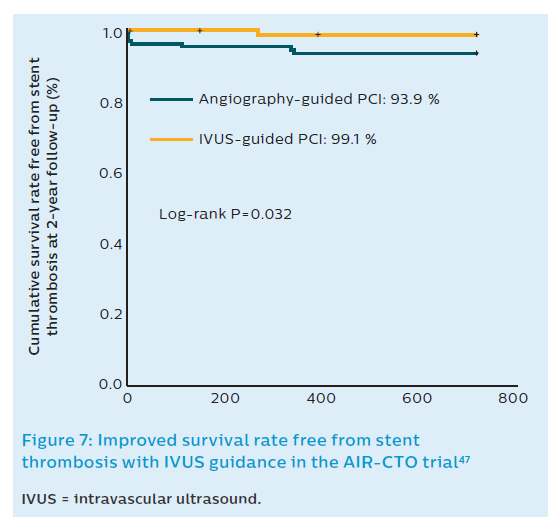
The treatment of CTOs involving long lesions is not seldom complicated by negative remodelling distal to the occlusion posing the operator with a dilemma regarding the extent of stenting. Stent deployment in this negatively remodelled segment may lead to different problems. On the one hand, stent oversizing resulting from eyeballing stent dimensions may cause vessel rupture. On the other, deployment of stents in a segment that is likely to undergo flow-induced remodelling after opening the CTO may limit this salutary process, or even lead to long-term ISA.46 The AIR-CTO study randomised 230 patients with CTO lesions to IVUS-guided DES implantation versus angiography-guided implantation.47 Using IVUS, a greater minimal lumen diameter was achieved with lower late lumen loss (0.28 mm versus 0.46 mm; P=0.03) and lower rate of in-stent restenosis (3.9 % versus 13.7 %; P=0.02) at 1-year angiographic follow-up.47 In this study, the proportion of second-generation DES was 27.8 % in the IVUS-guided group (n=115) and 20.0 % in angiography-guided group (n=115; Figure 7). Although there is no consensus on how to manage this situation, a conservative approach when IVUS reveals negative vessel remodelling distal to a CTO is recommended.
Bioresorbable Polymeric Scaffolds
Biosresorbable vascular scaffolds (BVS) have been proposed as a valid alternative to stents in diffuse vessel or long stenoses. Scaffolds offer several theoretical advantages over permanent metallic caging of the vessels especially when long lesions need to be treated. Temporary scaffolding might allow long-term restoration of endothelial structure and function, without affecting future surgical coronary revascularisation attempts and shows late lumen gain. Yet, emerging evidence from registries and pooled trial data suggest that the risk of BVS thrombosis may be higher than DES when incomplete apposition or underexpansion occurs. As these factors are more likely to occur in long lesions, IVUS can be of particular help both in selecting BVS size and guiding the PCI procedure. Recent 2-year follow-up data from the ABSORB II study have shown worrying rates of MI (7 % versus 1 % with everolimus-eluting stent [EES]; P=0.06) and also an unexpected risk of late scaffold thrombosis.48 To date, no studies on the translation of IVUS guidance of BVS implantation to clinical outcomes are available.
Stent Overlapping
Further to the discussion about stent sizing earlier on, recently, longer DES (32 mm) have become available, and it is occasionally difficult to decide whether to use a long single stent (LSS) or overlapping double stents (ODS) for diffusely diseased segments. LSS is superior to ODS in terms of medical expenses and nonoverlapping sites, but inferior in terms of stent delivery in vessels with severe calcification and tortuosities. Evidence suggests that EES provides similar angiographic and 1-year clinical outcomes regardless of whether one stent is used or two stents are overlapped.49 Similar results were obtained in the Gauging coronary Healing with biOresorbable Scaffolding platforms in Europe (GHOST-EU) trial with bioresorbable scaffolds.50 Another important consideration in this regard alluded to above is the maximum expansion of stents. In the setting of diffusely diseased segments, where there may be a considerable change between distal and proximal reference segments, it may recommended to use two adequately sized stents rather than one long stent, sized on the distal reference. A stent selected based on the distal luminal diameter may not be expandable to the proximal luminal reference without causing stent fracture. Therefore, using two stents, one sized based on the distal reference and one on the intended proximal reference, may lead to a better long-term result.
In-stent Restenosis
Intracoronary imaging may play an important role in evaluation of underlying mechanical factors that contribute to in-stent restenosis (ISR).51,52 For example, IVUS readily detects the presence of neointimal hyperplasia obstructing the stent, device underexpansion, or edge problems. In addition, the external elastic lamina is usually well delineated behind the stent struts, and this information provides potentially valuable insights on vessel sizing for optimisation of stent expansion.53 The practice of selecting a stent for the treatment of ISR based on the dimensions of the previously deployed stent ignores the fact that undersizing and underexpansion are likely causes of the stent failing in the first place. Only with intravascular imaging techniques can such a situation be addressed. This is all the more important in cases of diffuse ISR segments. The use of co-registration can be particularly helpful in this scenario. In cases where restenosis occurs at a site where overlapping stents are present, ballooning with a drug-eluting balloon may favour adding another layer of stent struts. The European Society of Cardiology clinical practice guidelines have echoed the potential value of IVUS in the management of stent failure with a class IIa, level of evidence C recommendation. Drug-eluting balloons may have a role in the treatment of ISR, especially where a second or third layer of stent struts is undesirable or where bleeding risks make the shorter duration of dual antiplatelet therapy favourable. However, the Restenosis Intra-Stent of Drug-Eluting Stents: Drug-Eluting Balloon vs Everolimus-Eluting Stent (RIBS IV) study showed that the use of drug-eluting balloon when compared with EES was associated with less favourable clinical and angiographic outcomes.54
Conclusions
Diffuse stenoses represent a subset of lesions that have retained high rates of stent failure. Relying on angiography alone can lead to inadequate procedural actions, which may effect short-term and long-term outcomes of PCI. IVUS helps both in planning and guidance of PCI in these lesions, and complements physiological information obtained with pressure vessel mapping. In the absence of trial-based algorithms to use IVUS in this context, we propose a structured approach to obtain and apply IVUS-derived information to address relevant matters that have been shown to play a role in short- and long-term procedural failure.
Case study #1
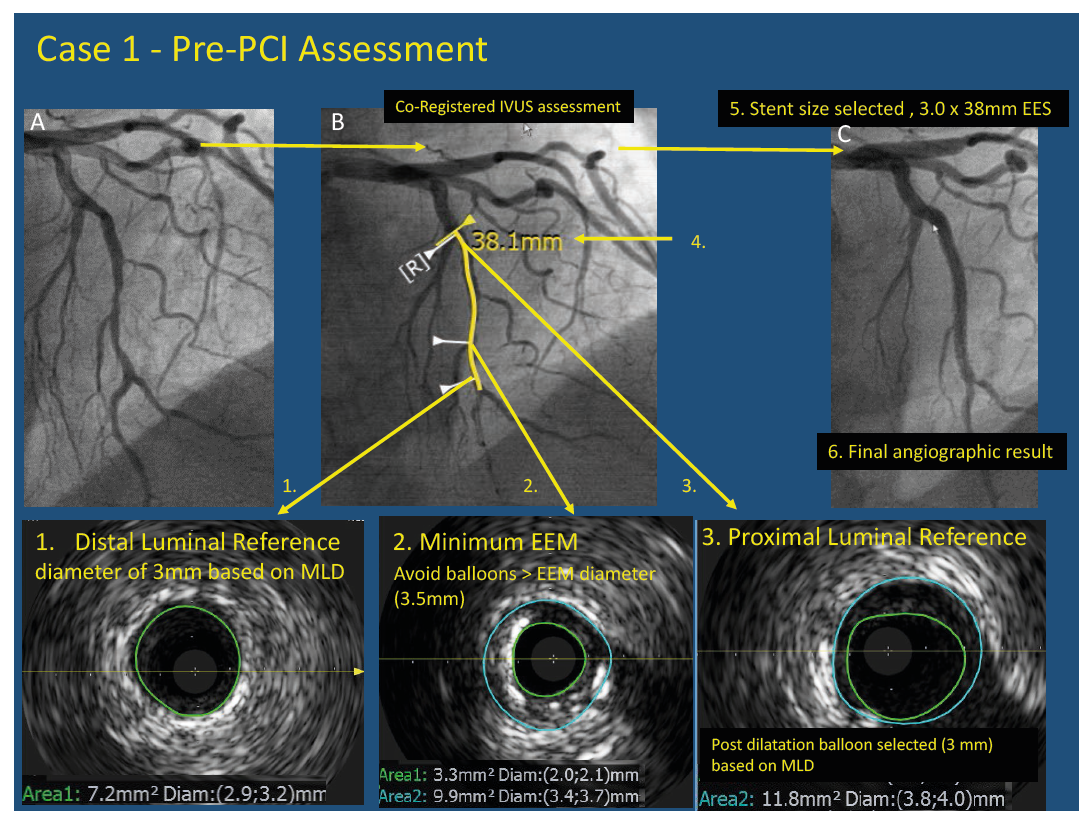
Pre-PCI Assessment stent sizing. Pre-PCI assessment of long LAD lesion with IVUS showed the selection of the distal luminal reference (1) in a zone with little atherosclerotic burden. The proximal reference was selected with <50% atherosclerotic burden, at the level of the septal branch, the bifurcation was chosen as a landing zone for the stent as this would allow the achievement of greater minimal stent area. The proximal reference luminal diameter (3) was used to select post dilatation balloon size. Care was taken not to use balloons or stents larger than the minimum EEM diameter (2). A 3.0 x 38mm EES was deployed with NC balloon post-dilatation with a 3 x 10mm balloon. A good final angiographic result was obtained (6)
Case study #2
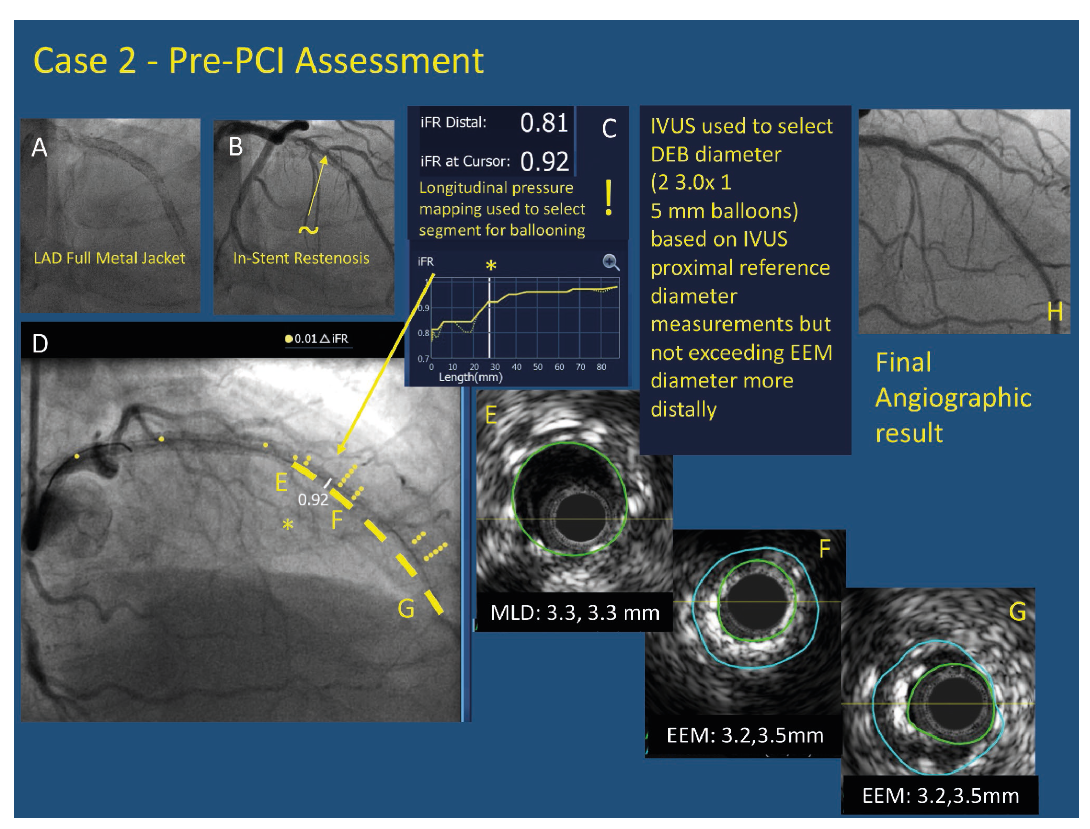
Pre-PCI assessment balloon sizing in a patient with a pre-existing full metal jacket in the LAD (A) with significant in-stent restenosis (~) on angiography (B). iFR pullback with co-registration demonstrated that largest drop (step) in iFR (*) on both longitudinal pullback (C -right) and co-registered pullback (D -left) occurred in mid-distal segment while there was another important drop further distally. The largest drop in pressure occurred at an area of stent underexpansion (F). The proximal reference segment (E) was used to select drug eluting balloon size. The length to be treated targeted the areas of most haemodynamic significance on pullback and the areas showing underexpansion on IVUS. Final angiographic result is seen (H)
Case study #3
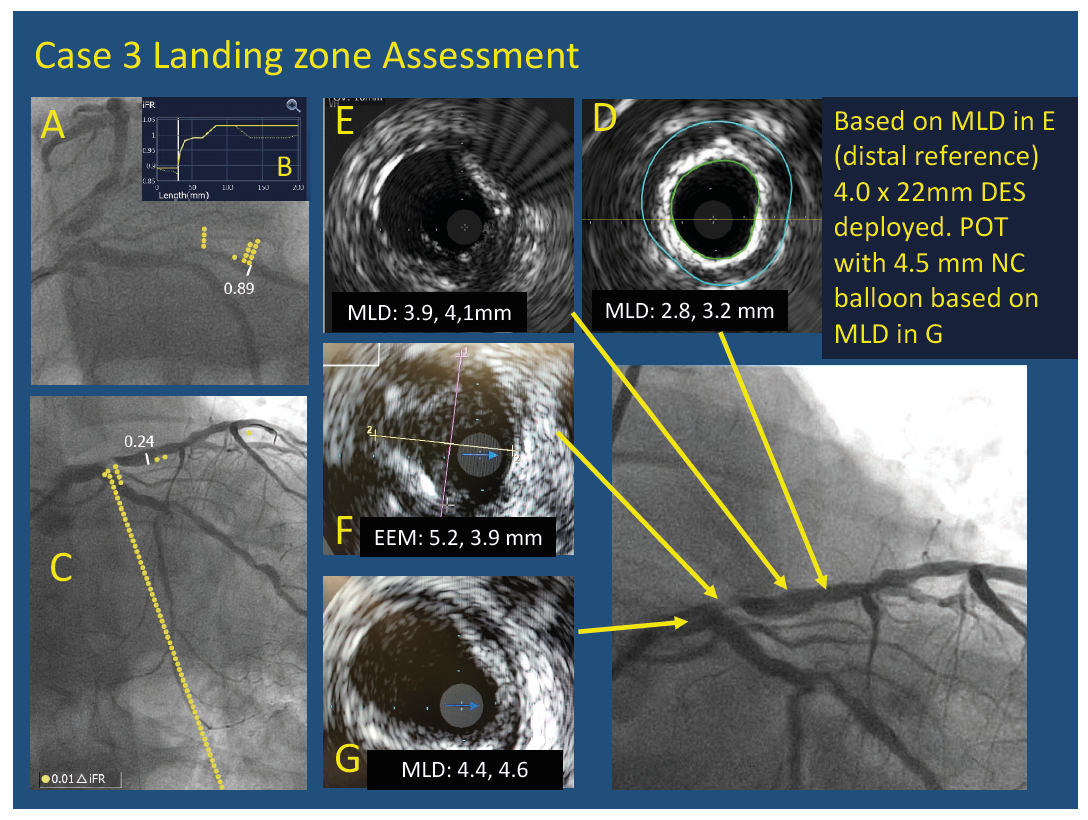
Example of assessment of stent landing zone in a patient with an ostial LAD stenosis. Physiological assessment showed iFR of 0.89 in the circumflex shown co-registered with angiography (A) and on longitudinal pressure mapping (B). iFR co-registration of 0.24 in the LAD (C). In the LAD the pressure drop was localised to the ostium; however, a short stent could not be deployed because of the absence of an adequate landing zone. This focal lesion is found to be diffuse angiographically. IVUS pullback from LAD showed that the best distal landing zone (E) had a plaque burden of 40 % with a reference diameter of 4 mm. There was no adequate landing zone at the LAD ostium (F) and the EEM was found to be more than 4 mm. A proximal landing zone was chosen in the distal LM (G) and a 4 x 22 mm stent was deployed using a T and provisional technique since the ostium of the LCX was free of disease. Proximal optimisation was performed using a 4.5 x 8 mm balloon with an excellent angiographic result.