Since the introduction of CRT more than 20 years ago, its role in mild to severe systolic heart failure has become well established. CRT has been shown to decrease mortality, reduce heart failure hospitalisations and improve functional status in patients with NYHA class II–IV heart failure and QRS prolongation, most commonly with LBBB pattern.1 One of the major limitations of CRT implementation is the significant number of ‘appropriate’ candidates, as determined by guidelines, who fail to respond with clinical, functional or structural endpoints. The rate of non-responders has been estimated between 20 % and 40 %.2 Efforts to predict those patients who will respond to CRT and to optimise the magnitude of response have been important areas of focus with regard to the future of CRT. This article will present recent considerations and advances, both technical and theoretical, in CRT.
Background
Efficacy of CRT therapy in select patient populations has been demonstrated in multiple large, randomised clinical trials (RCTs). The first multi-centre, randomised trial to demonstrate clinical benefits of CRT was the Multisite Stimulation in Cardiomyopathy (MUSTIC) trial published in 2001.3 This trial examined 67 patients with LV ejection fraction (LVEF) ≤35 %, NYHA class III heart failure symptoms, sinus rhythm and QRS duration >150 ms who had biventricular pacemakers placed. The devices were initially programmed to ventricular back-up pacing for rates <40 beats per minute for a period of 3 months followed by reprogramming to encourage biventricular pacing. CRT resulted in significant improvement in 6-minute walk distance, quality of life and peak oxygen uptake as well as decreased hospitalisations. Eighty-five per cent of the study patients reported that they felt better during the period with biventricular pacing programmed on.
The Multicentre InSync Randomised Clinical Evaluation (MIRACLE) trial evaluated a similar but much larger patient population.4 There were 453 patients with NYHA class III–IV heart failure, LVEF ≤35 % and QRS duration ≥130 ms randomised to biventricular or no pacing. Over 6 months of follow-up, significant improvements were noted in 6-minute walk distance, NYHA class and quality of life. Additionally, CRT was an effective adjunct to optimal medical therapy in reducing the secondary combined endpoint of heart failure hospitalisation or death. Although the results of MUSTIC and MIRACLE were encouraging in terms of the clinical benefits of CRT, reduced mortality had not yet been proved.
The Comparison of Medical Therapy, Pacing and Defibrillation (COMPANION) trial used a combined primary endpoint of hospitalisation or death from any cause.5 Enrolling 1,520 patients with LVEF ≤35 %, NYHA class III–IV heart failure and QRS duration >120 ms, this trial randomised subjects to optimal medical therapy, medical therapy with a CRT pacemaker (CRT-P) or medical therapy with a CRT defibrillator (CRT-D). At 1 year of follow-up, the CRT-D group showed significant reduction in overall mortality versus medical therapy alone, and the CRT-P group had showed a strong trend for reduced mortality (p=0.059). These results suggested a mortality benefit from CRT even in the absence of defibrillator capabilities.
Several studies have since examined the effects of biventricular pacing alone (i.e. CRT-P) on heart failure. The Cardiac Resynchronisation in Heart Failure (CARE-HF)6 trial utilised a composite primary endpoint of all-cause mortality or hospitalisation for a major cardiac event and a secondary endpoint of all-cause mortality. A total of 813 patients were randomised to optimal medical therapy or CRT. All patients had LVEF ≤35 %, NYHA class III–IV heart failure, QRS duration ≥120 ms and echocardiographic evidence of ventricular dyssynchrony. CRT-P was associated with a statistically significant 26 % reduction in the composite primary endpoint after 29 months as well as significant reduction in the secondary endpoint. CARE-HF was thus the first trial to demonstrate a mortality benefit with CRT even in the absence of defibrillator therapy. Expanding the findings of these trials, the Resynchronisation-Defibrillation for Ambulatory Heart Failure (RAFT) trial randomised 1,798 patients with LVEF ≤30 %, NYHA class II–III heart failure and QRS duration ≥120 ms to CRT-D or implantable cardioverter- defibrillator (ICD) without CRT.7 RAFT showed an absolute 7 % reduction in all-cause mortality or heart failure hospitalisation in the CRT-D group, confirming benefit of CRT over traditional ICD therapy.
Another patient group of interest were those with only mildly reduced LVEF, also at times referred to as the mid-EF population. It was previously shown in the Dual Chamber and VVI Implantable Defibrillator (DAVID) trial8 that chronic right ventricular (RV) pacing worsens long-term ventricular function and patient outcomes. The Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK-HF) trial9 evaluated patients with NYHA class I–III heart failure but required LVEF only ≤50 %. CRT-D was utilised only if an indication for defibrillation existed, otherwise CRT-P devices were implanted. Patients were randomised to standard RV or biventricular pacing. The major finding of the BLOCK-HF study was that over 37 months of follow-up, patients assigned to biventricular pacing had a significantly lower rate of the combined endpoint of death from any cause, urgent visits for heart failure and increase in LV end-systolic volume index, demonstrating the negative effects of chronic RV-only pacing. These results were similar in both CRT-D and CRT-P groups. The inclusion of an LV remodelling measure in the composite primary endpoint was controversial, but statistically significant reductions in the other clinical components were also observed.
Published in 2009, the Multicentre Automatic Defibrillator Implantation Trial – Cardiac Resynchronisation Therapy (MADIT-CRT) was the largest CRT trial to date and evaluated subjects with milder (NYHA class I–II) heart failure with a reduced EF.10 Among 1,820 patients with LVEF ≤30 % and QRS duration ≥130 ms, randomisation to biventricular pacing with a CRT-D device reduced the combined endpoint of mortality and heart failure events (defined as need for intravenous diuresis) by 29 % versus no pacing but ICD backup. Although the majority of the benefit was in reducing heart failure events, this trial showed that NYHA class I–II patients could also derive benefit from CRT.
The Resynchronisation Reverses Remodelling in Systolic Left Ventricular Dysfunction (REVERSE) study was performed concomitantly with MADIT CRT. This was a double-blind, multinational, randomised trial that enrolled patients with an LVEF <40 % and NYHA I–II CHF. All subjects received either a CRT-P or CRT-D depending on the local guidelines at the time. The primary endpoint was the clinical composite response (CCS) measured at 1 year of follow-up. The overall distribution was improved with CRT ON, although the proportion of patients who worsened with CRT did not differ.11 The pre-planned 5-year follow-up of the REVERSE study showed a survival benefit of CRT-D compared with CRT-P,12 extending earlier observations noted previously from the COMPANION study.
Guidelines
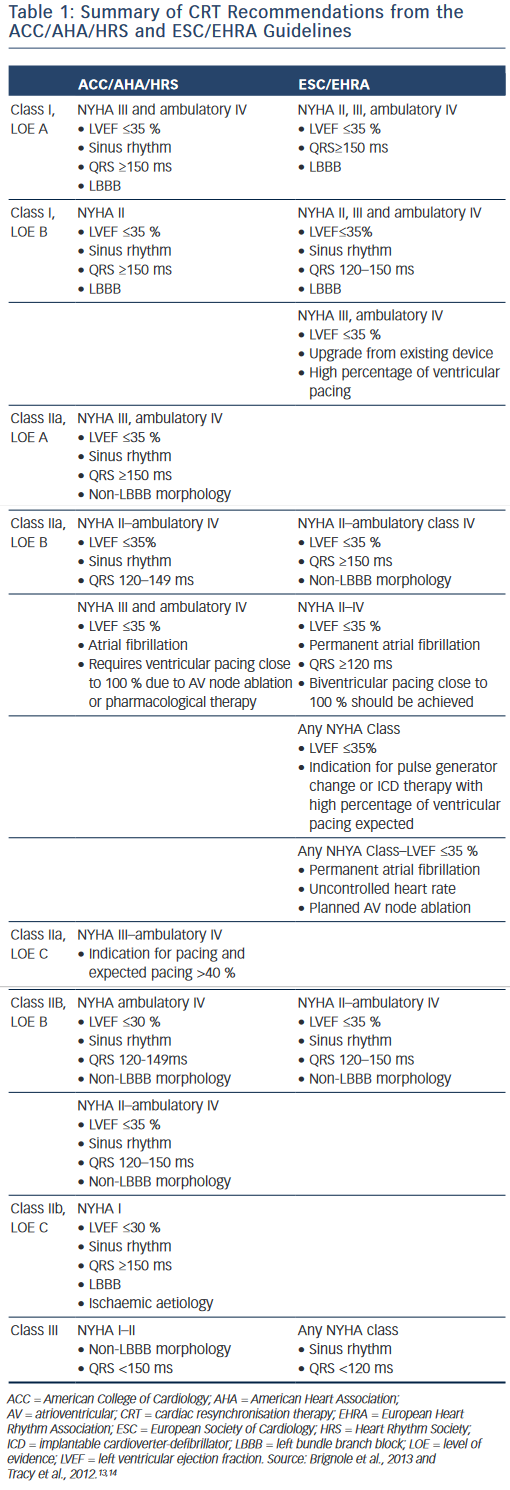
The results of the aforementioned trials, among others, led to publication of guideline recommendations on CRT by the major scientific societies.13,14 A consensus statement endorsed by the European Heart Rhythm Association (EHRA), Heart Rhythm Society (HRS), American College of Cardiology (ACC), American Heart Association (AHA), European Society of Cardiology (ESC) and Heart Failure Society of America (HFSA) was published in 2012 regarding preoperative evaluation and operative management.2 All patients considered for CRT are recommended to undergo careful pre-implant screening including comorbidities, routine labs, functional assessment, quality- of-life measurement, echocardiogram for quantification of LVEF and cardiac size, and electrocardiogram (ECG) to document QRS duration and morphology. Additionally, optimal medical therapy per current guidelines should be instituted and stable. Both the ESC/EHRA13 and ACC/AHA/HRS14 guidelines have class I recommendations for CRT-D in patients with NYHA II, III and ambulatory class IV heart failure with LVEF ≤35 %, sinus rhythm, QRS duration ≥150 ms and LBBB. Chronic RV pacing has also become an accepted indication for CRT. Additional recommendations are summarised in Table 1.
New Developments in Cardiac Resynchronisation Therapy
As both CRT-D and CRT-P have become broadly accepted, attention is now turning to improving rates of response and how this goal can be achieved. Ideally CRT patients will have ventricular pacing as close to 100 % of the time as possible.15 Recent areas of interest for maximising CRT response include patient selection, optimised and individualised lead placement, device programming and new technologies for delivering CRT.
Patient Selection
As demonstrated by subgroup analyses of the studies reviewed above, the best outcomes with CRT were observed among patients with very prolonged QRS duration (> 150 ms) and LBBB. However, guidelines recommend less strongly expansion of CRT to include patients with less severe heart failure and some QRS morphologies other than LBBB.
Both MADIT-CRT10 and the REVERSE trial11 included low percentages of patients with NYHA class I heart failure (15 % and 18 %, respectively). Neither of these trials demonstrated a significant advantage in terms of patient outcomes in this population, although REVERSE did show improved ventricular size and function in CRT patients. Solomon et al., also as part of the MADIT-CRT trial, did show evidence of LV reverse remodelling in these patients.16 A later post hoc analysis of MADIT-CRT was able to demonstrate the NYHA class I patients with ischaemic aetiology and LVEF ≤30 % had a 53 % relative risk reduction in heart failure events or death versus ICD only.17 No benefit was observed in non-LBBB patients. This finding prompted the ACC/AHA/HRS guidelines to add a class IIb recommendation for CRT-D in patients with NYHA class I heart failure, QRS duration ≥150 ms, LBBB and ischaemic aetiology.14 Currently, this is the only indication for CRT in NYHA class I patients; however, with expanding utilisation in this patient population other subgroups who might benefit are likely to emerge.
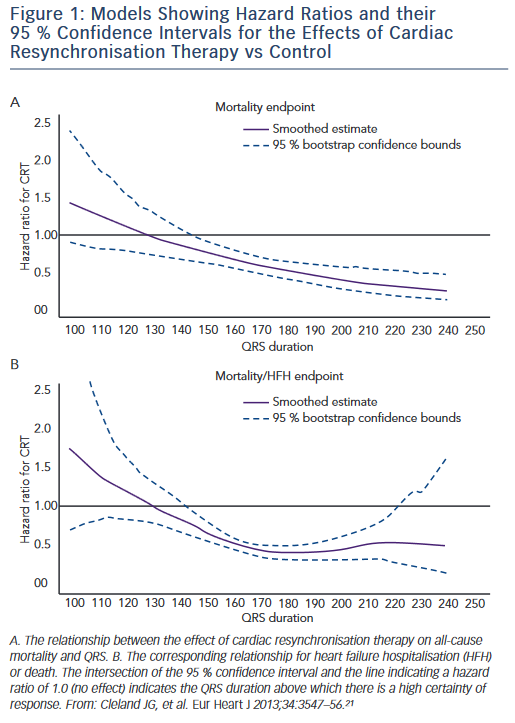
CRT therapy has been suggested for patients with QRS prolongation and non-LBBB morphologies, but trial data are mixed in this regard. An analysis of data from the MIRACLE and Contak-CD trials showed no improvement in symptoms, 6-minute walk distance or quality of life scores after 6 months of follow-up in RBBB patients.18 Both MADIT- CRT and RAFT increased heart failure event-free survival rates in LBBB patients but not those with right bundle branch block (RBBB) or intraventricular conduction delay (IVCD).16,19 However, the REVERSE trial showed a benefit of CRT among patients with RBBB and mild HF.20 In addition, a large individual patient meta-analysis of multiple randomised trials showed that QRS duration was the best predictor of clinical response with CRT independent of QRS morphology (see Figure 1).21 Given these conflicting results, interest remains to better identify non- LBBB heart failure patients who will best benefit from CRT. In this regard, pacing at sites of late mechanical or electrical delay may be particularly important for these lower response candidates as discussed below.
QRS duration that best predicts CRT response also remains an active area of interest. Randomised trials have consistently shown maximum benefit from CRT in those with QRS duration ≥150 ms.11,16,19 More recently the trend has been to apply the ≥150 ms cutoff more strictly for patients with lesser degrees of heart failure (NYHA class I/II) and non-LBBB QRS morphologies, while pursuing CRT for patients with QRS duration >120 ms for patients who are more ill (NYHA class III/ambulatory class IV).22
Individualising Lead Placement
LV lead implantation via the coronary sinus (CS) is the established technique for transvenous implementation of CRT. Lateral or posterolateral LV position has long been preferred; the results of large multicentre studies have shown that apical positions are the anatomic site associated with worst outcomes.23,24 This technique for CRT delivery has been limited by individual patients’ unique anatomic variations with regard to distribution and size of CS branch vessels.
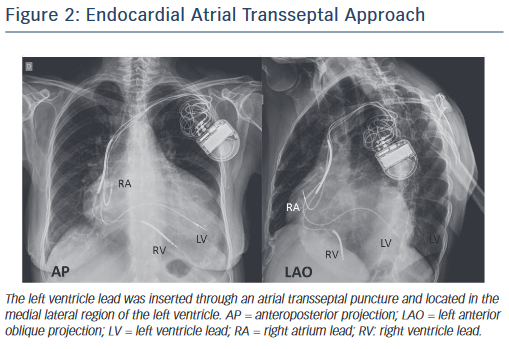
The only alternative to transvenous CS cannulation has been surgical placement of epicardial LV leads, an approach with significantly increased risk due to the invasive nature of the procedure. Recently, an endocardial approach using a transseptal puncture and with a lead crossing the mitral valve has been explored (Alternate Site Cardiac Resynchronisation [ALSYNC] trial), but this is still considered investigational (see Figure 2).
In addition to the use of multipolar leads, attention has been turned towards optimising traditional lead placement. Several approaches have been explored, including identifying areas of LV scar, assessing the latest mechanical or electrical LV activation and new techniques for delivery, including multi-site LV pacing.
Mechanical Synchrony
Despite general assumptions regarding LV lead placement on the lateral/posterolateral wall, optimal placement in fact varies between patients due to differences in underlying heart disease and site of maximal electrical and/or mechanical delay.25 Echocardiographic imaging, most recently using speckle tracking, has been used to identify sites of latest LV mechanical activation. In the Targeted Left Ventricular Lead Placement to Guide Resynchronisation Therapy (TARGET) trial, Khan et al. performed speckle tracking radial strain imaging on 220 patients prior to undergoing CRT implant.26 Patients were then randomised to standard LV lead placement or implant at the latest site of mechanical activation as identified by echocardiogram. The latter group demonstrated a significantly greater response rate at 6 months (70 % versus 55 %; p=0.031) as defined by reduction of ≥15 % in LV end-systolic volume and greater reduction in NYHA class. The Speckle Tracking Assisted Resynchronisation Therapy for Electrode Region (STARTER) trial used similar techniques to optimise LV lead placement and demonstrated a greater event-free survival (hazard ratio 0.48; p=0.006).27 Although these trials demonstrate the promise of this approach to CRT delivery, the increased time associated with screening in this manner and the percentage of patients who cannot undergoing speckle-tracking imaging due to inadequate image quality (11 % in TARGET)26 have limited its utility. Additionally controversy exists regarding whether speckle tracking is the ideal measure of latest site of mechanical contraction. Thus, although promising as a means to increase CRT response rates, this technique requires further study.
Scar Imaging
Imaging, to identify both location and extent of transmural scar, has been suggested as a means to predict clinical response and guide lead placement. Those with greater scar burden are less likely to show a clinical response to CRT28 and patients with non-ischaemic aetiology of heart failure show greater degrees of improvement in LVEF and reverse remodelling compared with ischaemic cardiomyopathy.4 Both single-photon emission CT and, more recently, contrast-enhanced MRI have been used to identify areas of transmural scar. Presence of scar in the posterior–lateral LV, the region typically targeted for LV lead placement, was shown to reduce rates of clinical response and reverse remodelling of the LV in several trials.29–32 An LV lead with its tip in such an area of scar is less likely to effectively pace the LV.33 Despite these findings, pre-implant cardiac MRI remains uncommon practice due to expense, inconvenience and inability to perform in patients with an existing pacemaker or defibrillator.
Electrical Dyssynchrony
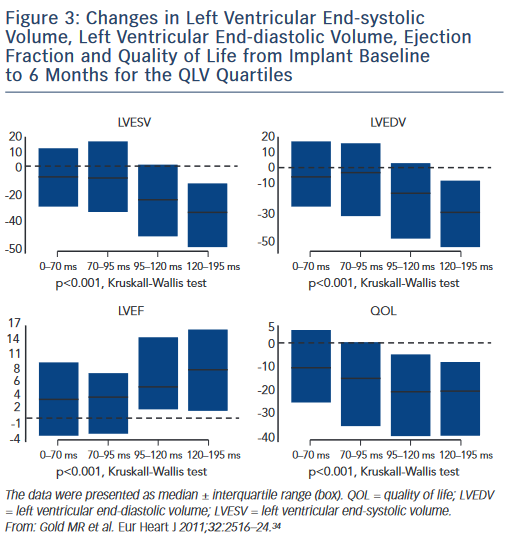
Another recent area of study is targeting the area of latest electrical activation, most commonly assessed by the QLV interval. Defined as the time from onset of QRS on surface ECG to the first large peak of the LV electrogram (EGM),34 this measurement is obtained at time of CRT implant. Among 426 patients, Gold et al. observed that lead placement in the highest quartile of QLV measurement (QLV ≥120 ms) was strongly associated with both echocardiographic improvement (reduction in LV end-systolic volume) and improved clinical outcomes as measured by quality-of-life questionnaires (see Figure 3). These results were confirmed by a substudy of the SmartDelay Determined AV Optimisation (SmartAV) trial, which showed longer QLV durations were strongly associated with positive reverse remodelling response to CRT (≥15 % reduction in LV end-systolic volume)35 and even greater improvement was observed with long QLV and optimised AV delays. Other recent trials have shown that the longest QLV intervals (>95 ms) are associated with greater acute haemodynamic improvements (LV dP/dt max).36 Thus targeting the site of latest electrical activation, as defined by a QLV interval greater than the median of 95 ms, can improve patient response to CRT. Subgroup analyses from these trials show that this is a robust predictor of response, even among subjects with non-LBBB, ischaemic aetiology or QRS duration <150 ms. The ratio of QLV to QRS duration has also been used as a measure of LV electrical delay and this predicts CRT outcomes.37
Multi-site Left Ventricular Pacing
Conventional delivery of CRT involves pacing the LV from a single site. Small trials have suggested that CRT nonresponders might benefit from placement of an additional LV lead to achieve multi-site LV pacing. So-called triventricular pacing, referring to the number of sites of stimulation, may be useful, especially in those with enlarged left ventricles and intraventricular conduction delay.22 A small randomised trial of 54 patients with conventional indications for CRT performed by Lenarczyk et al. showed significant improvements in NYHA class, VO2 max and 6-minute walk distance and response rate in triventricular versus standard CRT groups.38 Another small trial demonstrated benefit of the triventricular strategy in patients with permanent atrial fibrillation.39 These studies did not observe significant increases in procedural time, fluoroscopy exposure or complications associated with implantation of an additional LV lead. In 2012, Ginks et al. examined LV endocardial and multi-site pacing in 10 patients with standard indications for CRT.40 The patients first underwent complete electrophysiology study including non-contact mapping of LV endocardium for areas of scar. Response to conventional CRT pacing was then compared with LV endocardial and multi-site pacing. Acute haemodynamic improvement, as measured by change in LV dP/ dt, showed a 26 % increase from baseline with standard biventricular pacing, 37 % increase with LV endocardial pacing (p<0.0005 compared with standard biventricular pacing) and 47 % increase with triventricular pacing (p=0.08 compared with LV endocardial pacing). Thus, there was a statistically significant improvement with LV endocardial pacing and a trend towards even further improvement with multi-site pacing. Although this study was too small to identify differences between patient groups, the authors do note that those patients with myocardial scar and less QRS prolongation responded better to LV endocardial and triventricular pacing. This study did not utilise clinical endpoints and was also limited by the requirement for invasive testing prior to device implant. Pappone et al. found improvement in LV dP/dt and reduction of QRS duration by 22 % with multi-site LV pacing in 2000,41 and more recently has shown that multiple other haemodynamic metrics also improve.42 Generally lacking in the studies of triventricular pacing, however, is proof of clinical benefit. Based on these promising pre-clinical results there are two ongoing randomised trials, Triple-Site versus Standard Cardiac Resychronisation Therapy (TRUST CRT)43 and Dual Site Left Ventricular Pacing (DIVA), for which results are not available. As yet there are no results from a large RCT that prove the benefit of this approach. The Dual Site LV Pacing in CRT Non-Responders (V3) trial currently underway will enrol 84 patients at multiple French hospitals classified as non-responders to CRT and randomise to implant of an additional LV lead or continuing standard CRT.44 The primary endpoint of this study will be heart failure clinical composite score at follow-up. Moreover, it will also be important to compare these results with the simpler approach of multi-point pacing from a quadripolar lead as described below.
Technological Advances
After appropriate patient selection, the other major area of focus for decreasing CRT non-responder rates is optimal lead placement, including the use of novel technologies to improve CRT delivery. These new techniques represent hope for the effective delivery of CRT in the 5–8 % of patients in whom a LV lead cannot be delivered and the 15–20 % in whom the lead position is suboptimal.45 Several exciting new technologies have recently become available commercially or are being studied with promising results and these will be reviewed briefly here.
Leadless Left Ventricular Pacing
Although not yet introduced commercially, a system for leadless LV endocardial pacing is being developed and studied. The WiCS-LV system (EBR Systems, Inc.) is currently undergoing safety and feasibility trials.
This system uses ultrasound waves to pace the LV endocardium using acoustic energy. A small electrode is implanted in the LV endocardial wall via a retrograde aortic approach and a pulse generator is implanted subcutaneously in an overlying intracostal space.46 The system senses the RV pacing from a concurrently implanted traditional dual chamber pacemaker or defibrillator and triggers LV stimulation from the RV pacing spike. Ultrasound pulses delivered from the pulse generator to the endocardial electrode to induce LV contraction. In the Wireless Stimulation Endocardially for CRT (WiSE-CRT) trial, Aurrichio et al. reported 1- and 6-month results after implanting this device in 17 heart failure patients of three types: those with failed traditional LV lead implantation via the CS, non-responders with a functional LV lead and patients with a dual-chamber device, but no previous attempt at LV lead placement.47 System implant was successful in 13 (76.5 %); complications included failure of LV capture in one (5.8 %) and pericardial effusion in three (17.6 %). The authors report a significant increase in LVEF at 6-month follow-up, shortened QRS duration at 1 and 6 months and improvement of at least one NYHA functional class in two- thirds of patients. Although the results of this small trial are promising as an alternative to traditional LV lead placement, they also demonstrate safety and efficacy concerns of a new device that require further study before widespread utilisation can be considered.
Alternate Approaches to Left Ventricular Lead Placement
Other techniques for LV endocardial pacing have also been studied. As discussed above, the ALSYNC trial is the first large prospective multi- centre trial of LV endocardial pacing. However, earlier pilot studies were performed. Betts et al. describe a series of 10 patients with either failed CS lead placement or nonresponders due to suboptimal lead positioning in whom LV endocardial leads were placed using transseptal puncture of the interventricular septum.48 This technique, performed via the subclavian vein, required coronary angiography and ventriculography prior to the procedure. An active fixation lead was then delivered to the LV lateral endocardial wall successfully in nine of 10 patients reported. This technique has the obvious disadvantage of requiring lifelong anticoagulation to avoid thrombus formation on the LV lead. Response to LV pacing was generally good with eight out of nine patients improving at least one NYHA functional class and five out of nine showing decrease in LV end-systolic volume and increase in LVEF. Complications included failure to capture at 3-month follow-up, VT during procedure requiring external cardioversion and VT storm necessitating emergency cardiac transplant. This technique is undergoing further study in the Interventricular Septal Puncture for Cardiac Resynchronisation Therapy to Treat Heart Failure (LV-CONSEPT) trial. The atrial transseptal approach has also been reported;49–51 however, this technique has been limited by technical difficulties with the procedure. Overall, although LV endocardial pacing is promising for those with those patients in whom LV lead placement is not possible or CS anatomy does not provide an optimal branch location there has not yet been a system or technique for delivery that can allow its widespread utilisation.
Quadripolar Pacing Leads
A significant development in delivery of CRT that is commercially available in the US is the quadripolar LV lead. Utility of traditional bipolar LV leads has been limited by high pacing thresholds and phrenic nerve stimulation resulting from limited potential pacing vectors. Quadripolar LV pacing leads have now been introduced by St Jude Medical, Medtronic and Boston Scientific. These leads expand pacing options relative to bipolar leads by providing between 10 and 17 potential vectors.52–54 Increasing options for pacing vectors creates greater ability to achieve LV capture at lower threshold and to avoid phrenic nerve stimulation by ‘programming around’ these complications. An additional advantage of quadripolar LV leads is their availability with several lead tip shapes (straight, straight with tines and fixed ‘S’ or hook-type curves) for greater lead stability and reduced chance of lead dislodgement. Phrenic nerve stimulation has been reported in up to 20 % of CRT patients with bipolar leads,55 a complication that frequently results in the LV lead being programmed ‘off’, thus eliminating potential for CRT delivery. A multi-centre registry of quadripolar LV leads published in 2014 found that among 721 patients receiving LV leads (347 quadripolar; 364 bipolar), more patients experienced phrenic nerve stimulation with quadripolar (16 %) versus bipolar (11.6 %) leads.56 Device reprogramming using alternate pacing vectors, however, eliminated phrenic nerve stimulation in all patients with quadripolar leads but only 60 % (24/40) with bipolar leads. Lead repositioning was necessitated in those with bipolar leads in whom phrenic nerve stimulation was not remedied by device reprogramming, thus exposing them to the inherent dangers of repeat invasive procedures. This registry also demonstrated lower rates of lead dislodgement with quadripolar (1.7 %) compared with bipolar (4.6 %) leads. Finally, the pacing threshold at implant was significantly lower with quadripolar leads, potentially prolonging pulse generator battery life. Although this study did not find a significant difference in successful implant rates, the relative larger size of quadripolar leads means not all CRT patients will have CS branch vessel anatomy suitable for delivery. Recent observational data have suggested a mortality benefit from the use of quadripolar leads, although the mechanism of this benefit is not yet defined.56
Device Programming
Improved algorithms for device programming demonstrates promise in improving CRT delivery. In addition to increasing the percentage of paced beats, these algorithms have focused on atrioventricular (AV) and interventricular (VV) interval optimisation, which have the potential to improve CRT response rates and possibly increase the magnitude of response.28 AV optimisation aims to ensure complete LV filling prior to contraction; VV optimisation attempts to minimise ventricular mechanical dyssnchrony.57 AV and VV optimisation is speculated as having a particularly important role in improving haemodynamic response when LV lead positioning is not considered optimal.58 Techniques for AV optimisation traditionally have used echocardiographic parameters, including measuring aortic and mitral velocity-time integral with multiple AV intervals,59 but these methods are costly and time consuming. Greater attention is now being paid to automatic AV and VV optimisation through device-based algorithms. Device manufacturers have developed proprietary algorithms, including Smart AV Delay (Boston Scientific),60 QuickOpt (St Jude Medical)61 and AdaptiveCRT (Medtronic).62 Smart AV Delay considers intrinsic AV intervals, intraventricular timing and LV lead location, and it is designed to achieve fusion between intrinsic conduction through the interventricular septum and paced activation of the latest activated region of the LV.63 QuickOpt uses the duration of right atrial contraction to set AV delay such that ventricular contraction occurs fully after atrial depolarisation and contraction are complete, setting the paced AV delay as sensed AV delay plus 50 ms.61 AdaptiveCRT calculates AV delay from intracardiac EGMs to fuse LV pacing with intrinsic contraction.62 An active trial evaluating a novel pacing algorithm designed to optimise haemodynamic function weekly is underway.64 Compared with echocardiographic optimisation or fixed AV delays, neither QuickOpt (in the Frequent Optimisation Study Using the QuickOpt Method [FREEDOM]) nor Smart AV Delay (in the SMART-AV trial) improved heart failure measures (reverse remodelling and symptoms). AdaptiveCRT was found to have non-inferior outcomes compared with echocardiography-based optimisation of CRT,62 although this trial does not necessarily present a ‘real world’ comparison as echocardiographic CRT optimisation is uncommon in practice. Although as a whole these trials have not shown significant improvement in heart failure outcomes compared with standard device programming this remains an area of active research. The ongoing Clinical Trial of the SonRtip Lead and Automatic AV–VV Optimisation Algorithm in the PARADYM RF SonR CRT-D (RESPOND- CRT) randomises patients to the Sorin SonR CRT optimisation algorithm or the control arm (echocardiographic optimisation).19,64 The primary effectiveness endpoint is based on proportion of responders to CRT therapy at 12 months. Secondary endpoints include freedom from death or heart failure hospitalisation, worsened NYHA class and lead electrical performance. The Adaptive CRT algorithm is also being tested in a prospective study of LBBB and normal AV conduction time, which is the group that benefited most from this therapy.35 Finally, patients with a prolonged QLV interval were shown to benefit from the SMART Delay algorithm.62 Thus, although currently available evidence does not suggest benefit from routine AV and VV optimisation there may remain a role for this practice in select patients.
Conclusion
CRT is an important and well-proven procedure for the management of patients with heart failure and QRS prolongation. The impact of CRT, however, has been limited by inadequate delivery of and unpredictable response to therapy. Recent efforts have focused on patient selection, individualising LV lead placement and novel technologies to improve therapy response rates. Ongoing research and development efforts hope to broaden indications for CRT by improving prediction algorithms for CRT response and by delivering therapy in optimal fashion.
Clinical Perspective
- Cardiac resynchronisation therapy (CRT) is an important management strategy for patients with New York Health Association (NYHA) class II–IV heart failure and left bundle branch block (LBBB) whose indications are expanding.
- New technologies, including leadless and multi-site pacing, offer hope for reducing the high (20–40 %) rate of non-responders to CRT.
- Individualised left ventricular (LV) lead placement to reduce mechanical and/or electrical dyssynchrony may also have a role in optimising delivery of CRT.