Leaflet motion abnormalities (LMAs) are a relatively new entity in the field of transcatheter aortic valves (TAVs).1–4 They can be associated with the thrombosis of the bioprosthesis (TAVT), often leading to dramatic clinical scenarios1 or, on the other hand, they can result in a hypoattenuated leaflet thickening (HALT) and/or reduced leaflet motion (RELM), both usually associated with gradients within the normal range.2–4 We hereby discuss the potential screening process and subsequent management of LMAs and their possible associated clinical transcatheter aortic valves conditions, with a specific focus on the role of the imaging modalities.
Incidence of Transcatheter Aortic Valve Implantation With Thrombosis of the Bioprosthesis
Most of the cases of TAVT reported in the literature presented with recurrence of symptoms at follow-up, non-ST-elevation myocardial infarction, embolic events (such as stroke) or cardiac arrest.5–9 Possible treatments for TAVT include anticoagulation, TAV implantation (TAVI)- in-TAVI and surgical aortic valve replacement (SAVR).5,6,8,10 In recently published registries, the cumulative incidence of symptomatic TAVT ranged from 0.61 to 2.8 %.1,10 Almost all patients in the registries presented with worsening shortness of breath, and elevated transaortic gradients were detected in the majority of the patients (24 of 26 [92.3 %]). TAVT presented as thickened leaflets or thrombotic apposition of leaflets in 76.9 % and thrombotic mass on the leaflets in 23.1 %. Anticoagulation was successful in 23 of 26 patients (88.4 %), the remaining patients have been treated with transcatheter valve-in-valve procedure or surgical aortic valve replacement.
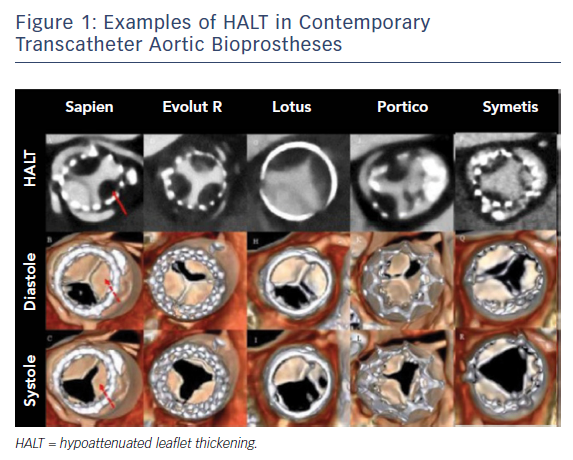
The prevalence of HALT/RELM has been reported in three studies.7 Makkar et al. reported findings from 55 patients using 3D volume-rendered (VR) imaging.2 RELM was noted in 39 of 187 (20.9 %) patients and in multiple transcatheter valve types, including the Portico™ valve (St. Jude Medical); SAPIEN, SAPIEN XT and SAPIEN 3 (Edwards Lifesciences); CoreValve™ (Medtronic) and the Lotus™ Valve System (Boston Scientific).
Pache et al. performed contrast computed tomography (CT) in 156 patients undergoing TAVI with the SAPIEN 3 valve at a median of 5 days post TAVI; HALT was noted in 16 (10.3 %) patients.3 Leetmaa et al. performed CT in 140 patients with SAPIEN XT valves within 3 months post implantation.4 TAVT (defined as HALT) was present in five patients (4 %), four of these patients being asymptomatic with no echocardiographic evidence of significantly elevated gradients.
Echocardiography
Transthoracic echocardiography (TTE) plays a crucial role to exclude regurgitation and/or stenosis, but it provides inadequate details to assess the possible presence of HALT/RELM. Of note, the presence of HALT/RELM, is usually associated with ‘normal’ gradients. It is thus conceivable that after calculating normal gradients (which are usually higher than the native valve) even an expert echocardiographer may not carefully look for HALT/RELM. The latter issue may imply that the real incidence of this phenomenon is far from being precisely depicted.
In some occasions, the transoesophageal echocardiogram (TEE) may be helpful to detect the RELM, before or after the CT scan findings; however, it is impractical to advocate the use of TEE in all cases, especially when the TTE shows normal gradients and no suspicious findings.
Computed Tomography
All acquisition protocols enabling the formal assessment of leaflet motion and thickening employs contrast CT with retrospective gating.
The acquisition is usually performed in the craniocaudal direction from the aortic arch to the diaphragm and images reconstructed at 0.6 mm slices with 0.3 mm overlap and iterative reconstruction for evaluation at 10 % intervals within the 0–90 % RR range. To minimise radiation exposure a dose-modulation approach can be used, thus the reducing dose in the 55–100 % RR range (diastole).
CT images are usually reconstructed in the systolic phase using 3mensio Valves™ version 7.0 or 7.1 (3mensio Medical Imaging BV), and Vitrea® Software Version 6.7.2 (Vital Images, Inc.). The valve leaflets can be assessed using both 2D (axial cross-section assessment) and 3D-VR imaging. The VR images can be generated using centreline reconstructions and the hockey puck feature in 3mensio or using front-cut plane or 5 mm thick slab VR functions in Vitrea. In Vitrea, the medium de-noising filter was employed.
Of note, while leaflets with normal motion are difficult to visualise on 4D VR-CT, leaflets with reduced motion can be clearly seen in 3D or 4D images. Hypo-attenuating lesions can be studied on maximal intensity projection (MIP) 2D CT and correlated to reduced leaflet motion on 3mensio software with the use of the marker feature and on Vitrea software using the VR auto-alignment with MIP feature.
Theoretically, the CT-scan acquisition and reconstruction can be deemed as the gold-standard imaging tool to visualise the leaflets; however, it provides no haemodynamic information. Thus, the CT scan is actually complementary to the TTE/TEE, although in all the published series on this topic, the CT scan has been used to confirm the diagnosis.
Echocardiography Versus Computed Tomography
A discrepancy must be acknowledged between CT and echocardiographic findings. Despite a 10–15 % prevalence of subclinical thrombosis with CT, elevated gradients (a mean gradient of >20 mm Hg) with echocardiography are infrequent.1–6 This observation implies that CT detects early subclinical thrombosis, whereas echocardiography detects the late consequences of thrombosis – i.e. valvular stenosis. This also indicates that not all thromboses result in valve degeneration, i.e. early thrombosis might resolve spontaneously.11
Dynamic 4D CT imaging has consistently been used for detection of subclinical thrombosis, although consensus of definitions and quantification of leaflet thrombosis with CT is lacking and should be established before prospective studies and clinical practice are carried out.
Moreover, the CT timing after TAVI to detect meaningful leaflet thrombosis is unknown. It has been postulated that the timing of imaging might affect the proportions of leaflet thrombosis with different valve types (see Figure 1);8 however, there is no evidence to support a risk linked to a specific type of bioprosthesis.
Impact of HALT/RELM on Clinical Events and the Role of Anticoagulation
Leetmaa et al. did not observe any cases of HALT (TAV thrombosis) in patients on anticoagulation.4 Makkar et al. reported similar findings in patients on anticoagulants compared with patients on antiplatelet therapy,2 and initiation of anticoagulation following detection of HALT/ RELM resulted in resolution of the imaging findings and of the LMA in all patients.2–4
Of note, with respect to the presence of leaflet thrombosis and possible subsequent clinical events, a discrepancy is noted between the 10–15 % prevalence of CT thrombosis and the proportion of 3–4 % of patients with stroke in large clinical trials. Moreover, Chakravarty et al. reported that, in addition to subclinical leaflet thrombosis being less common in patients receiving warfarin or non-vitamin K antagonist oral anticoagulants than in those receiving antiplatelet agents, the thrombosis resolved in all 36 patients who were given anticoagulants, but persisted in 20 of 22 (91 %) patients not receiving anticoagulants.7 The fact that HALT/RELM is seldom observed in patients on anticoagulants and often resolves with initiation of anticoagulation suggests that this finding is related to TAVT. Thus, it may be reasonable to perform a CT scan in selected clinical situations (dysfunctional TAVI, worsening heart failure, stroke/transient ischaemic attack, myocardial infarction or other clinical situations suggesting embolic phenomena) and patients on anticoagulants with symptomatic TAV thrombosis (HALT/RELM).
On the other hand, given the risks of chronic anticoagulation, questions remain:
- Should all patients be offered such therapy?
- Should patients be selected according to imaging findings?
- What is the optimal duration of treatment?
- With new oral anticoagulants being considered to be preferable over vitamin K antagonists, how and when should we re-assess the efficacy of the treatment?
Moreover, predictors of subclinical TAV thrombosis (including the propensity of individual valve types) are unknown.
Conclusion
Before robust evidence that the imaging finding of HALT/RELM alone is clinically relevant becomes available, the management of patients with TAVI should not change. Both the European Society of Cardiology and American College of Cardiology/American Heart Association guidelines provide a Class IIb recommendation for dual antiplatelet therapy, but do not recommend routine anticoagulation.12,13 It is also wise to embrace the US Food and Drug Administration perspective that, based on findings to date regarding reduced leaflet motion, stated that the overall benefit–risk balance for use of TAVI remains favourable when they are used for their approved indications.9