Introduction
The difficulties observed in the management of coronary bifurcation disease in the early years of percutaneous coronary intervention (PCI) with plain balloon angioplasty triggered the development of techniques to improve procedural success and long-term outcomes. Coronary bifurcation lesions can account for up to 30% of lesions in patients with multivessel disease (MVD), so it is not surprising that coronary bifurcation lesions are a matter of vivid discussion and debate. This is fuelled by new definitions, classifications, measurements and imaging methods, as well as new devices including dedicated bifurcation stents.1
The relationships between diameter and flow in the coronary tree and between diameter, length, flow, and perfused myocardial mass are well known.2 Therefore, a coronary bifurcation is not merely comprised of the main vessel (MV) and a side branch (SB). Rather, it possesses three segments with different diameters and flows, i.e. proximally (the MV), distally (the main branch; MB) and, as already mentioned, a SB.
Several formulae have been proposed to describe these relationships. The three most popular are:3
- Finet’s formula: MVdiameter = 2/3 * (MBdiameter + SBdiameter)
- Murray’s law: MVdiameter3 = MBdiameter3 + SBdiameter3
- Huo-Kassab formula: MVdiameter7/3=MBdiameter7/3 + SBdiameter7/3
Low wall shear stress (WSS) is an established risk factor for development of the atherogenesis process. It is especially frequently observed in coronary bifurcations where low WSS is accompanied by slow and turbulent – or even reversed – flow.4 The bifurcation carina is an exception to this, where the WSS is high. Moreover, atheroma progression is triggered by flow disturbances and the initial plaque extends anterograde and circularly. This circular atheroma extension might explain that, in the case of large plaques, the carina might be affected in as many as on e-third of cases.5
The Medina classification is used for lesion assessment. It describes the disease presence in each segment of the coronary bifurcation (MV, MB and SB). In the classification, ‘1’ defines a lesion ≥50% diameter stenosis and ‘0’ as <50% stenosis. It has been shown recently that Medina’s classification is significantly affected by the imaging modality, whereby coronary CT angiography (CTA) improves the reproducibility of Medina classification compared with invasive coronary angiography.6
Left Main Peculiarities
Significant unprotected left main (LM) coronary artery disease is present in <10% of patients undergoing coronary angiography. In autopsy research, a mean LM length of 10.8 mm ± 5.2 mm (range 2–23 mm), mean LM diameter 4.9 mm ± 0.8 mm and mean angle between the left anterior descending (LAD) and left circumflex (LCx) of 86.7° ± 28.8° has been described. This angle value positively correlated with LM length.2 Further studies showed that long LM developed stenoses more frequently near the distal bifurcation compared to near the ostium (77% versus 18%).7 It is also worth emphasising that LM bifurcation disease is rarely focal and that both sides of the carina are almost never disease-free. Furthermore, continuous plaque from the LM into the proximal LAD artery has been reported in 90% of cases.8 Summarised below are the most crucial LM peculiarities (in comparison with non-LM bifurcations), which should be taken into consideration when distal LM stenosis PCI is planned:
- This is the only coronary bifurcation in which the MV originates directly from the aorta. It is associated with the possibility that guidewires can go behind the LM stent struts and there is a risk for longitudinal stent compression with intensive guiding catheter manoeuvres.
- Not rarely, the proximal reference diameter in LM may exceed 5.5–6 mm, which is near the dilatation limit of many contemporary coronary stents. Moreover, excessive heavy postdilatation may lead to strut fractures and affect prognosis.
The LCx is predominately the SB. This SB frequently has an extensive reference diameter and supplies a large myocardial territory. In consequence, acute occlusion may result in profuse and detrimental ischaemia. Furthermore, the take-off is frequently angulated, making it difficult to access with guidewires. This angle may also hamper the proper two-stent implantation technique.
Initial Diagnostics and Ischaemia Confirmation
Fractional Flow Reserve and Non-hyperaemic Pressure-derived Indices
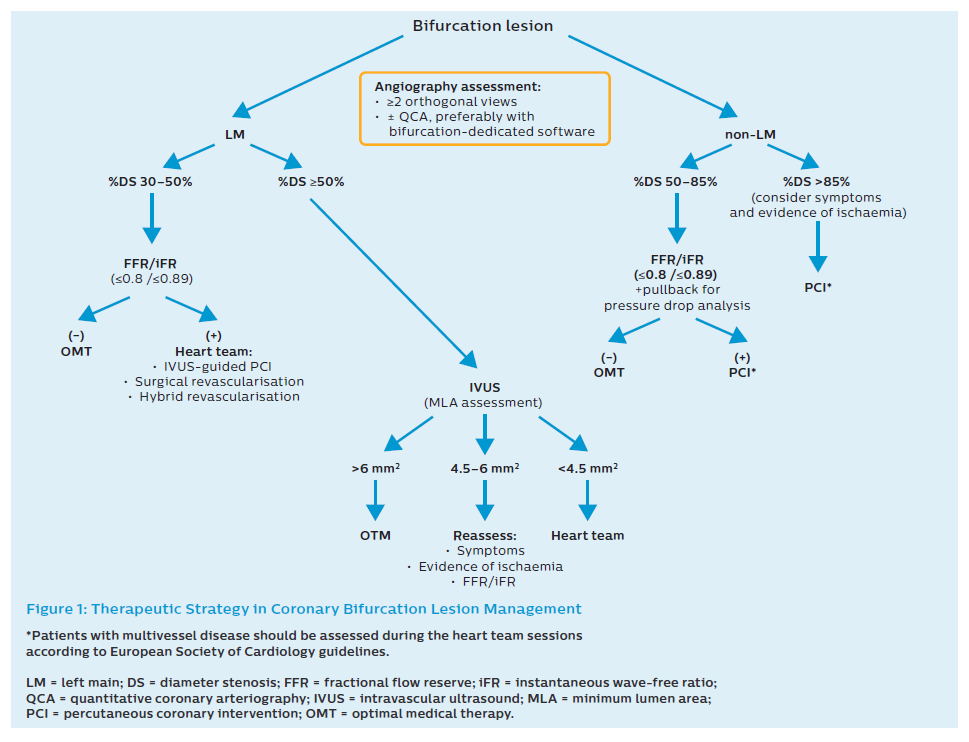
Fractional flow reserve (FFR) is an easily feasible modality for the physiological evaluation of stenosis in an epicardial coronary artery. FFR includes the interaction between the anatomical stenosis and perfusion area supplied by a specific coronary artery. A pressure wire can be applied to determine whether PCI is indicated both in the MB and SB. This technique is not recommended to decide whether a single-stent or two-stent approach is required before PCI.9 Nevertheless, after stent implantation in the MV–MB, a pressure wire can be used when ostial pinching in the SB is visible in the angiogram. Then FFR can be applied to verify that a second stent to the SB is unnecessary.10 For physiological assessment, non-hyperaemic pressure-derived indices such as instantaneous wave-free ratio (iFR) can be applied in coronary bifurcation lesions.11 The initial strategy is presented in Figure 1.
Left Main Assessment
FFR use in LM stenosis is more challenging because of the need for disengagement of the guiding catheter and the inability to administer adenosine intracoronary. Evaluation should include measurements performed in both the LAD and in LCx, including a pullback measurement. Recently there has been a surge of interest in resting indices such as iFR. Two large clinical trials – DEFINE-FLAIR and iFR-SWEDEHEART – showed non-inferiority of iFR-guided PCI to FFR-guided PCI.12,13 Recently Warisawa et al. showed in an observational study (DEFINE-LM) that proceedings in patients with LM disease based on iFR are safe and feasible.14 Other studies, such as POLBOS LM (NCT03508219) are on-going.15 Despite this it must be noted that iFR in LM has not yet been assessed in randomised trials and therefore that validation of this technique in LM assessment is needed. Nevertheless, the level of evidence granted by European Society of Cardiology guidelines for both FFR and iFR in the LM setting is the same.16
Intravascular Ultrasound
Intravascular ultrasound (IVUS) has transformed our comprehension of coronary atherosclerotic disease and the response to PCI. Notably, IVUS revealed that disease advancement is mostly more diffuse, and calcifications much more frequent, than observed in coronary angiography. The modality enables assessment of real reference vessel diameters and has legitimised the high-pressure deployment era. Therefore, it should be used in all cases where there are uncertainties – either during or at the end of the procedure. Also, the need for a pullback from both branches is underlined.17,18 The use of IVUS has been supported in recent European bifurcation guidelines.9,19–21 In the case of non-LM bifurcations, there are no strict and proven cut-off values.
Nevertheless, in the study by Won et al., long-term event rates were relatively low with pharmacotherapy without any PCI, so the threshold of IVUS-derived minimum lumen area (MLA) 4 mm2 may be too large to use for detecting significant stenosis in non-proximal epicardial coronary artery.22 Jang et al. appraised the diagnostic accuracy of IVUS-derived MLA compared with FFR to assess intermediate coronary stenosis. Their meta-analysis included 17 studies, comprising 3,920 patients with 4,267 lesions. The overall mean MLA threshold was 2.58 mm2. The pooled MLA sensitivity that predicted functionally significant coronary stenosis was 0.75 (95% CI [0.72–0.77]) and the specificity was 0.66 (95% CI [0.64–0.68]).23 However, the studies were not bifurcation-dedicated, and one should always consider the obtained MLA value alongside the patient’s symptoms and myocardial burden at risk.
In short, IVUS in coronary bifurcation enables the following:
- measurement of a true lumen and vessel (external elastic membrane dimensions in the MV and MB);
- analysis of atherosclerotic plaque burden (PB), morphology, and longitudinal distribution;
- stent landing zone and reference analysis;
- stenosis severity and negative remodelling assessment (mainly at ostial location);
- measurement of the lesion length (only during automatic pullback); and
- detection of angiographically silent disease (LM stenosis, a diffuse disease in reference segments).24
Sakamoto et al. proved that the MV plaque thickness on both walls of SB at the carina and SB diameter ratio (defined as ostial SB total diameter [media-to-media] divided by ostial SB luminal diameter [intima-to-intima]) were independent predictors of SB occlusion just after stent implantation.25
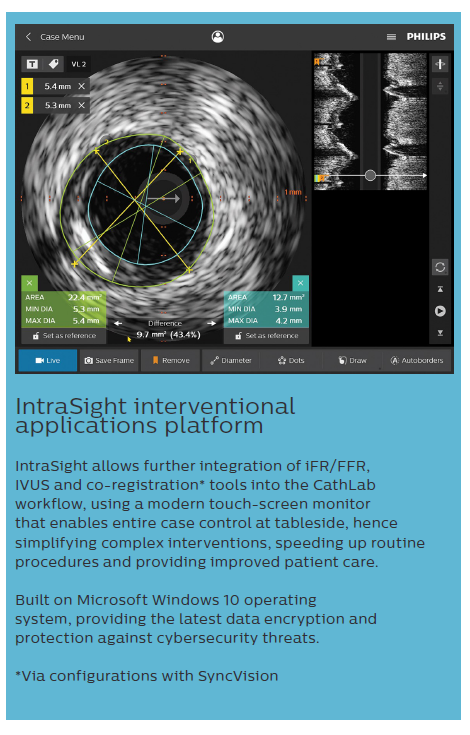
We should also mention available software to co-register both angiography and IVUS (or iFR). This facilitates the decision on the stented area and predicts the outcome after PCI.26
Intravascular Ultrasound in Left Main
Many trials have confirmed the applicability and the impact of IVUS on long-term outcomes in patients undergoing PCI in LM. A large study assessing 1,670 patients with LM lesions treated with drug-eluting stents (DES) demonstrated that IVUS-guided PCI was associated with a significant decrease in major cardiovascular events (cardiac death, MI, or target lesion revascularisation [TLR]) within 36 months (11.3% versus 16.4%, p=0.04).27 A trend for lower mortality was shown in the MAIN-COMPARE study, but without a difference in MI or TLR.28 A meta-analysis of 10 studies recently demonstrated a significantly decreased risk of all-cause death, cardiac death, and stent thrombosis in IVUS-guided LM PCI. Compared with angiography-guided PCI, IVUS-guided PCI statistically significantly lowered the risk of all-cause death (RR 0.60; 95% CI [0.47–0.75]; p<0.001), cardiac death (RR 0.47; 95% CI [0.33–0.66]; p<0.001), TLR (RR 0.43; 95% CI [0.25–0.73]; p=0.002) and in-stent thrombosis (RR 0.28; 95% CI [0.12–0.67]; p=0.004).29 Because of these benefits, IVUS should be used in each case of LM PCI (Figure 1).
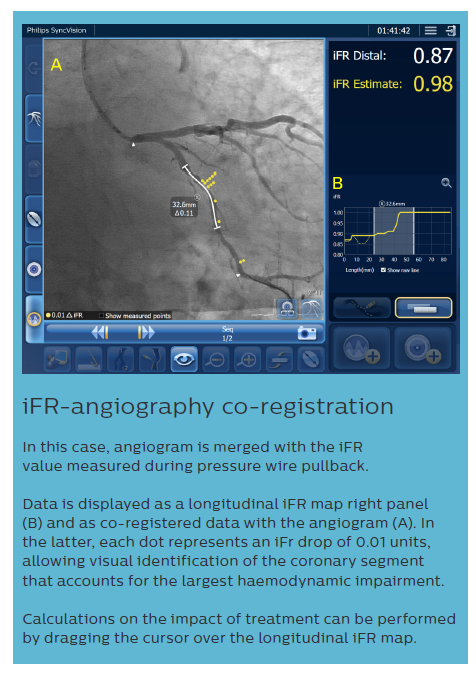
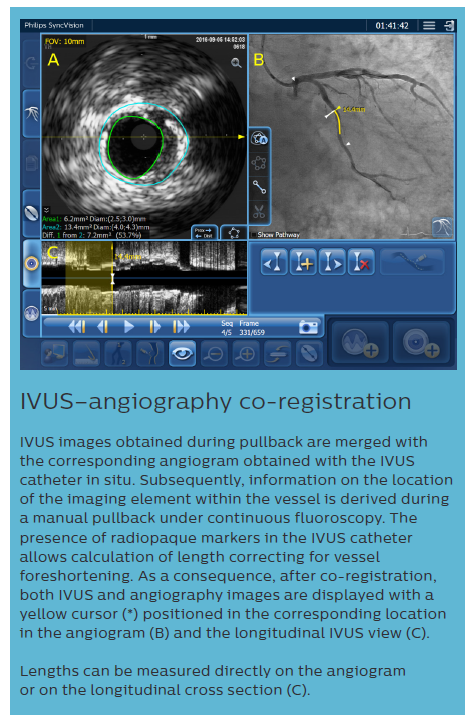
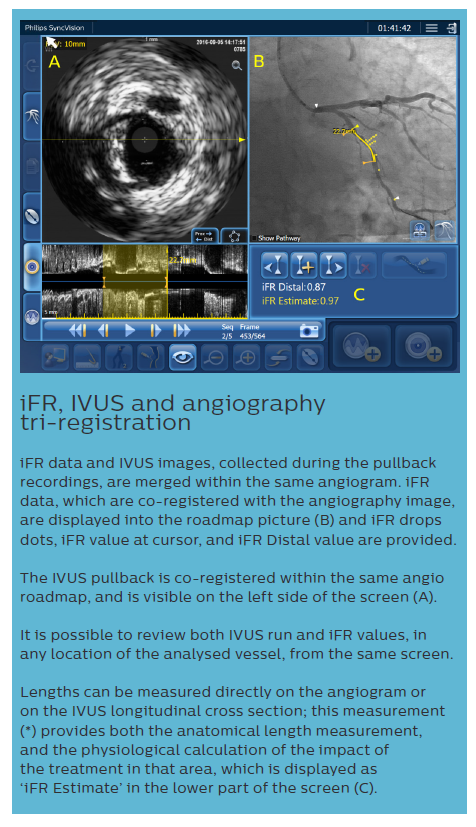
iFR data and IVUS images, collected during the pullback recordings, are merged within the same angiogram. iFR data, which are co-registered with the angiography image, are displayed into the roadmap picture (B) and iFR drops dots, iFR value at cursor, and iFR Distal value are provided.
The IVUS pullback is co-registered within the same angio roadmap, and is visible on the left side of the screen (A).
It is possible to review both IVUS run and iFR values, in any location of the analysed vessel, from the same screen.
Lengths can be measured directly on the angiogram or on the IVUS longitudinal cross section; this measurement (*) provides both the anatomical length measurement, and the physiological calculation of the impact of the treatment in that area, which is displayed as ‘iFR Estimate’ in the lower part of the screen (C). Based on European Bifurcation Club (EBC) consensus documents, the IVUS diagnostic approach should take into account the following considerations:9,19–21
- It is crucial to disengage the guiding catheter to avoid confusing the guiding catheter with ostial stenosis. It is essential to maintain a coaxial relationship between the IVUS catheter and the LM ostium.
- It is not reliable to use IVUS imaging of either the LCx or the LAD to infer the other vessel’s status tangentially during pullback, as either ostial lumen dimension or plaque burden assessment can be misleading.
- A discrepancy between the MLA in the LM when imaging from the LAD or LCx can be generated by the oblique plane of the IVUS beam when the transducer turns from the daughter vessel with the greatest angle into the LM.
In terms of cut-off values, LM sizing demonstrates less variability than other major epicardial vessels. Cut-off values of MLA <6 mm2 and <4.5 mm2 to predict functional impact have been validated with IVUS in Western and Asian populations, respectively. A LM IVUS-derived MLA >6 mm2 can be considered non-ischaemic while LM IVUS-derived MLA ≤4.5 mm2 can be considered ischaemia generating. A LM IVUS-derived MLA 4.5–6 mm2 suggests that additional invasive or non-invasive assessments of ischaemia are advisable. MLA measurement of non-LM lesions is not recommended for the assessment of lesion significances because of variation according to vessel calibre and subtended myocardium. Pressure-derived haemodynamic assessment is the gold standard for deferring revascularisation in patients with non-LM stable coronary artery disease.
There are several considerations when procedure planning with IVUS prior to stent implantation. First is assessment of the risk of SB compromise. Lesions proximal or distal to the SB or ostial SB stenoses affect the risk of SB compromise after MV–MB stenting. Patients who have a ‘vulnerable’ carina (the eyebrow sign) or significant calcium identified by IVUS longitudinal reconstruction are at particular risk of adverse carina shift towards the LCx. One must remember that SB compromise might be caused by plaque shift, but carina shift is probably more important.
Second and third are the determination of stent length and stent diameter. Using automated pullback, the proposed stent length can be measured to limit residual stenosis in adjacent segments (i.e. geographic miss). Segmental stent diameters can be based on proximal and distal reference size measurements (choice of distal reference only with further stent optimisation).
Fourth, reference size and length measurements can be used to plan the size and length of the proximal optimisation technique (POT) balloon catheter to ensure that it fits within the stent from the carina to the proximal stent edge.
Finally, IVUS studies in LM bifurcations have shown that a pre-procedural MLA of <3.7 mm2 and PB >56% at the LCx ostium predicted a post-stenting FFR <0.80 after LM bifurcation stenting with the cross-over technique.30
Optical Coherence Tomography
Optical coherence tomography (OCT) characterises at 10 times higher resolution than IVUS. It ensures less strut-induced artifact and enables precise evaluation of strut apposition. Presently, this is the best tool to guide and verify proper stent expansion. It might also be helpful in re-crossing into the jailed SB (distal strut) and in optimal stent positioning against the SB ostium.31 This is described in more detail in the recent consensus document prepared by Onuma et al.32 In summary:
- Online 3D reconstruction should be used wherever possible to gain the necessary insight into complex anatomy and device–vessel interaction during bifurcation.
- A 3D assessment of the ostial area from OCT pullback of the MV can be used in place of pullback from the SB.
- Use preprocedural OCT to assess plaque distribution, calcification and lipidic plaque to plan the stenting strategy; pay attention to a narrow carina tip – an angle <50° is associated with SB complication after MV stent implantation.
- Accurate vessel measurement is important in determining the choice of stent size and can be measured by either external elastic membrane area or lumen area. The latter is the preferred method in cases where the proximal vessel is too large to measure the external elastic membrane.
- According to the law of flow conservation, the stent’s size should be selected according to the law of flow conservation, aiming at the fractal geometry of bifurcation. It is recommended to select stent size based on the MB reference diameter and to allow for expansion to the MV reference diameter.
As far as the stent length is concerned, the operator should aim to cover the bifurcation stenosis segment at least 6–8 mm from the proximal stent edge to the bifurcation carina to enable subsequent POT with a short balloon.
Choosing the Optimal Treatment Strategy
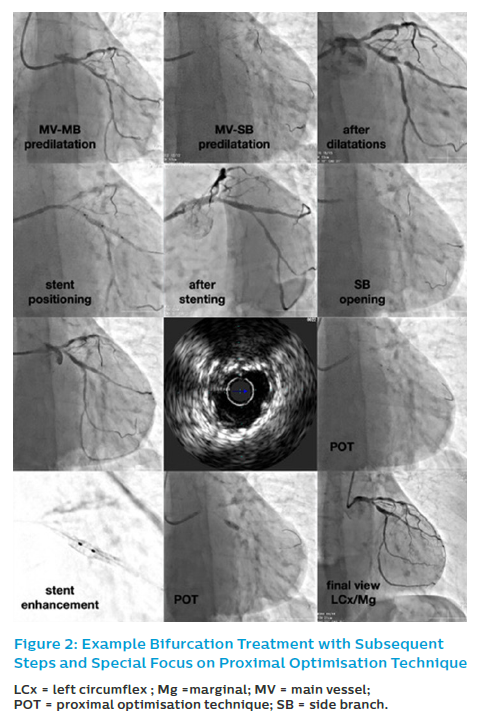
Provisional SB stenting is the standard strategy in most coronary bifurcation lesions (Figure 2). Almost all bifurcation lesions, including the distal LM, can be safely proceeded via the radial artery approach with a 6 Fr guiding catheter.9,19–21,33
Current guidelines on myocardial revascularisation recommend using DES in coronary bifurcation lesions. There are no specific guidelines in terms of qualification for treatment, PCI or coronary artery bypass grafting in the case of coronary lesions. Here, general rules for MVD and/or LM are applicable. However, mentioned guidelines give a Class I indication for PCI in patients with significant LM disease and a low/intermediate SYNTAX score. PCI in subjects with substantial LM disease and SYNTAX score ≥33 has a Class III recommendation.16
In their recent paper, Choi et al. suggest that – even in the second-generation DES era – the one-stent strategy should be considered the preferred approach for treating LM bifurcation lesions.34 The results were based on The Coronary Bifurcation Stenting registry III, in which 2,648 patients were enrolled. Median follow-up duration was 53 months. LM bifurcations were linked with a greater risk of target lesion failure (HR 1.84; 95% CI [1.31–2.58]; p<0.001) than non-LM bifurcations. The two-stent strategy was more common in LM bifurcations than in subjects with non-LM bifurcations (27.1% versus 11.7%; p<0.001). In LM bifurcations, compared with the one-stent strategy, the two-stent strategy disclosed a significantly greater risk of target lesion failure (two-stent versus one-stent, 17.4% versus 10.6%; HR 1.84; 95% CI [1.04–3.26]; p=0.035), mainly caused by the higher rate of TLR (15.3% versus 5.5%; HR 2.69; 95% CI [1.27–5.70]; p=0.009). The risk of cardiac death or MI did not differ between the two groups (4.4% versus 6.6%; HR 0.69; 95% CI [0.30–1.57]; p=0.38). For patients with a non-LM-bifurcation, there was no significant difference in the rate of target lesion failure between one-stent and two-stent strategies (5.6% versus 6.3%; HR 0.92; 95% CI [0.42–2.00]; p=0.84).
Side Branch Importance
The historical debate about how to assess the relative importance of an SB is still on-going. In the case of SB occlusion, minor troponin elevations may be safely ignored, but higher troponin levels are clinically significant, especially when leading to MI type 4a diagnosis. As a result, the SB length (<72 mm by angioCT), together with the SB stenosis characteristics (degree and length), should warrant the decision on SB protection during PCI. Moreover, an early decision to deploy two stents in these crucial SBs with complex/extensive stenoses (disease extending into the SB over 5 mm) is more justified, especially when both branches’ wiring is challenging.9,19–21
Recently, the visually estimated angiographic V-RESOLVE score was developed and validated as a simple and accurate invasive tool to predict the probability of SB occlusion after MV–MB stenting. It considers six risk factors: plaque distribution, MV TIMI flow grade before stenting, pre-procedural diameter stenosis of bifurcation core (%), bifurcation angle, diameter ratio between MV/SB and diameter stenosis of the SB before MV stenting.35
Moreover, recent evidence shows that CT may also help predict side branch occlusion.36,37 Grodecki et al. showed that Medina class with any proximal involvement of main vessel and SB (1.X.1) on CTA or invasive coronary angiography was the most predictive for SB occlusion during PCI with no significant differences between modalities (area under the curve 0.68 versus 0.66; p=0.693, respectively).6 Recently, Jeon et al. investigated the anatomical attributes determining myocardial territory of diagonal branches. They also tried to develop prediction models for clinically relevant branches using myocardial perfusion imaging and CTA.38 They proposed a decision tree model to predict the per cent fractional myocardial mass ≥10%. For example, they established that when there is non-dominant LCx and only one diagonal branch, this diagonal branch supplies blood to 17.6% of the myocardium.
Technical Aspects
Wiring the Bifurcation Lesion
Generally, the most challenging branch should be wired initially, among others, to avoid guidewire wrap. One should not push the guidewire in MB too distally to preserve the guidewire tip’s shape since it will be applied to guidewire the SB through the MV–MB stent. When SB wiring access is difficult, plaque modification with rotational atherectomy or occasionally balloon dilatation may facilitate the passage of a guidewire prior to stenting.
The EBC recommends the ‘jailing wire’ technique, which consists of leaving a guidewire in the SB while implanting a stent in the MB. It has the following potential advantages:
- The technique may preserve SB patency and – in case of occlusion – the guidewire shows the SB location.
- It facilitates access to the SB by favourably changing the angle of the bifurcation.
- The jailed guidewire is a kind of anchoring and provides more reliable support for the balloon to cross the SB ostium.
- In sporadic cases, the jailed guidewire can be applied as a rescue procedure, to advance a small balloon catheter and dilate the SB to restore the blood flow.9,19–21
Predilation
MB predilation facilitates the PCI procedure. It allows selection of the proper stent length required to promote a safe POT dilation. Routine SB predilation is not recommended. It may be considered when SB access is difficult, severe diffuse and/or calcified SB lesions are present, or when blood flow is compromised after wiring.9,19–21 Predilation can be performed both with semi-compliant as well as non-compliant (NC) balloon catheters depending on the plaque type, negative remodelling, and presence of calcifications.
IVUS and OCT are useful for detection, localisation, and quantification of coronary calcification. Diffuse target lesion calcification may negatively influence on PCI. It is associated with the ability of effective lesion dilatation and a higher likelihood of stent underexpansion and longitudinal stent deformation (LSD). In lesions with a circumferential ring of calcium >180° in IVUS, a more considerable calcific burden was linked with a smaller stent area and greater stent eccentricity.39 Here, we can use cutting/scoring balloons, as well as rotational atherectomy and/or intravascular lithotripsy.40
Beohar et al. examined outcomes according to lesion preparation strategy in patients from the EXCEL trial undergoing PCI in LM.41 The authors differentiated procedures into four groups: rotational atherectomy, cutting or scoring balloon, balloon angioplasty and direct stenting. The primary endpoint at 3 years comprised all-cause death, stroke, or MI. Among 938 patients, rotational atherectomy was performed in 6.0%, the cutting or scoring balloon in 9.5%, balloon angioplasty in 71.3% and direct stenting in 13.2%. In patients treated with direct stenting, balloon angioplasty, cutting or scoring balloon, and rotational atherectomy, respectively, there was a steady surge in SYNTAX score, LM complex bifurcation, trifurcation or calcification, number of stents, and total stent length. Procedural complications were observed in 10.4% in the whole population, with the lowest rate in the direct stenting group (7.4%) and the highest in the rotational atherectomy group (16.1%; p=0.22). The adjusted 3-year rates of major adverse cardiovascular events (MACE) did not differ according to the lesion preparation strategy. These findings might suggest that proper lesion preparation may be able to overcome the increased risks of complex LM lesion morphology.
Also worth mentioning is the paper by Kini et al.42 The authors examined the incidence of SB compromise after provisional stenting of calcified bifurcation lesions treated with rotational atherectomy or cutting balloon angioplasty and the utility of OCT to detect functionally significant SB stenoses. They disclosed that SB compromise rates and functionally significant stenosis after provisional stenting of calcified bifurcation lesions were similar between two lesion preparation strategies. The SB ostium area in OCT could have identified SB branches with FFR ≤0.80 with high sensitivity and specificity.
Main Vessel: Main Branch Stenting
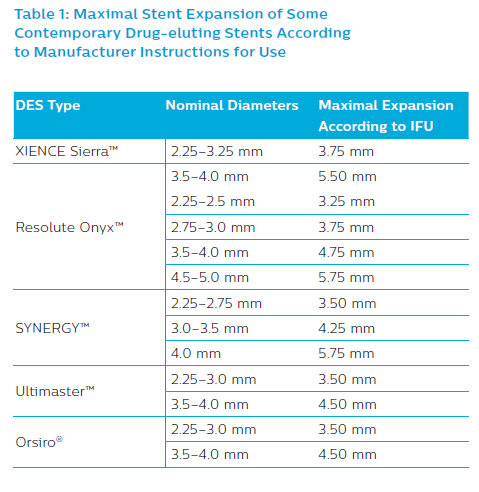
Second-generation DES are recommended. The selection of the most appropriate DES type must be made according to expansion ability to ensure optimal stent apposition in the MV and at the SB ostium. One may also consider dedicated bifurcation stents such as BiOSS LIM®/BiOSS LIM C® (Balton) or Tryton (Tryton Medical), as the two most thoroughly studied.43–48 The maximum stent cell diameter to be deployed over the SB ostium is also essential, especially in large arteries such as the LM. Stent diameter should be chosen according to MB size to reduce the risk of SB occlusion induced by carina shift and dissection within the distal bifurcation segment if the stent diameter is too large. When applying the distal sizing strategy, a final POT is essential to ensure optimal stent expansion (Table 1).9,19–21
Proximal and Distal Optimisation Techniques
POT should be performed routinely during the PCI as it optimises stent deployment/apposition and restores the vessels’ fractal geometry. It should be done before SB rewiring as it facilitates access to the SB and reduces the risk that the guidewire might cross into the SB behind the MV–MB stent, and it may also promote crossing into a distal cell within the MV–MB stent. POT may be repeated when the guidewire cannot enter the SB through the stent’s struts.
POT is performed by inflating a short, correctly sized balloon just proximally to the carina. Therefore, an additional 6–10 mm (the smallest length of commonly available balloons) needs to be considered when choosing the proposed MV–MB stent length. The diameter ratio between the balloon and the MV reference segment ought to be 1:1, and a NC balloon should be used. In the case of POT, exact placing of the balloon is vital. If it is too distal it poses the risk of SB occlusion and when it is too proximal it does not push the stent’s struts towards the SB ostium. The distal margin of the balloon must be located just proximal to the carina, whereas the proximal balloon should still be entirely within the stent to minimise the risk of proximal vessel injury.33
The technical challenges of POT include obtaining the accurate position of the balloon within the 3D geometry of the coronary bifurcation. Several stent enhancement techniques are now available. Also, the distal marker’s location compared to the distal margin of the balloon may vary between producers. When the balloon does not cover the entire stented MV area, it must be repositioned and inflated again to provide that the stent’s most proximal portion is well expanded.9,19–21 It was suggested in a post-hoc analysis of the EXCEL study that POT may not be always effective, so the correct choice of balloon catheter diameter and length based on IVUS/OCT is important.49
Analogous to this, the distal part of the stent might require optimisation in the MB. Such postdilatation is called the distal optimisation technique or Polish kiss, as used in the POLBOS LM study.50 Both proper stent expansion and apposition are crucial both in proximal as well as in the distal part of the bifurcation.43
Side Branch Treatment Opening and Final Kissing Balloon Technique
Removing a jailed guidewire from an SB can cause the guiding catheter to jerk forward into the vessel. Consequently, it is recommended to remove the guidewire in a steady, controlled manner to prevent guiding catheter traction. The risk of complications during trapped guidewire withdrawal can be minimised in calcified vessels by decreasing the length of a trapped guidewire behind the stent and lowering post-dilation pressures. Once the SB guidewire has been relocated through rather than behind the stent, further post-dilation can be safely performed. Both polymer-coated and non-polymer-coated guidewires can be safely jailed behind stents.
The provisional approach’s fundamental advantage is that SB treatment remains an alternative at any stage throughout the procedure. Opening the distal stent strut (close to the carina) of the MV–MB stent towards the SB improves the SB ostium’s scaffolding and diminishes the necessity for a second stent. To increase the chance of crossing the distal strut, the operator should have a perpendicular projection for the SB ostium and try to pull back the MV–MB guidewire (or a third guidewire in the same direction). If there are doubts or when a balloon will not cross, the jailed SB guidewire can be removed, and a further attempt can be made. The guidewire cross’s position and its position relative to the stent can be accurately assessed by OCT. Use of NC balloons is recommended as they are associated with a lower risk of SB dissection. Consequently, there is a lower requirement for an SB stent.9,19–21
In a small SB, a ‘keep it open strategy’ is usually the best approach, starting by wiring both branches and stenting the MB followed by POT. Unless flow is reduced within the SB, further treatment is unnecessary. In small SBs with reduced flow, rewiring through the stent struts, with SB dilation and additional final POT, should be initially considered. Even when kissing balloon inflations (KBIs) are performed, further final POT inflation is still recommended to minimise the eccentricity index and optimise around the MB lumen (KBI can result in an oval MB luminal contour). The decision to place a second stent is based on vessel size and the quality of the achieved result. However, ensuring an optimal outcome in the MB should be given priority over-optimising the SB appearance.
When the SB is significant, both branches should always be wired before dilation. Stent implantation in the MB and POT should be performed in sequence. At the 14th consensus meeting of the EBC, POT/side/POT or a POT/KBI/POT approach was debated. Preference was confirmed for the POT/KBI/POT strategy as it was less likely to cause problematic stent deformation when performed in a ‘real-world setting.’ However, clinical verification of this is still awaited.9,19–21
When performing KBI, experts suggest using short NC balloons – the MV balloon should be sized 1:1 according to the MB diameter. They also recommend, based on the bench test data, that one should ensure minimal balloon overlap in MV with sequential NC balloon inflation (alternate inflation of MV–MB and SB balloon followed by simultaneous inflation/deflation).43 In our research, we proved the importance of POT and KBI regarding long-term outcomes. Analysing restenotic cases from POLBOS I and POLBOS II trials, we found that POT and KBI were less frequently performed precisely in the restenotic lesions.51 In multivariate regression analysis, POT decreased the risk of MACE and TLR both at one year and at four years of follow-up. These findings were confirmed both for regular DES as well as for dedicated bifurcation stent such as BiOSS.45,52 The procedure in provisional T-stenting strategy and IVUS guidance is presented in Figure 2.
The modern stent’s design with thin struts made of cobalt alloy has improved trackability, conformability, and flexibility of many stents. However, their low longitudinal axial strength makes them prone to LSD. In the case of distal LM bifurcation with stenting from the ostium, one must be cautious and gentle to avoid LSD during final optimisation techniques and manoeuvring with a guiding catheter (e.g. guiding catheter tip during removal of jailed buddy wire). Nevertheless, it is worth remembering that other factors can also lead to LSD in non-LM bifurcations too, such as the passage of catheters like balloon catheter, IVUS catheter, guideliners, etc., a stent crossing itself through a calcified lesion or a passage of another stent through already implanted stent.53
Side Branch Treatment: Stenting
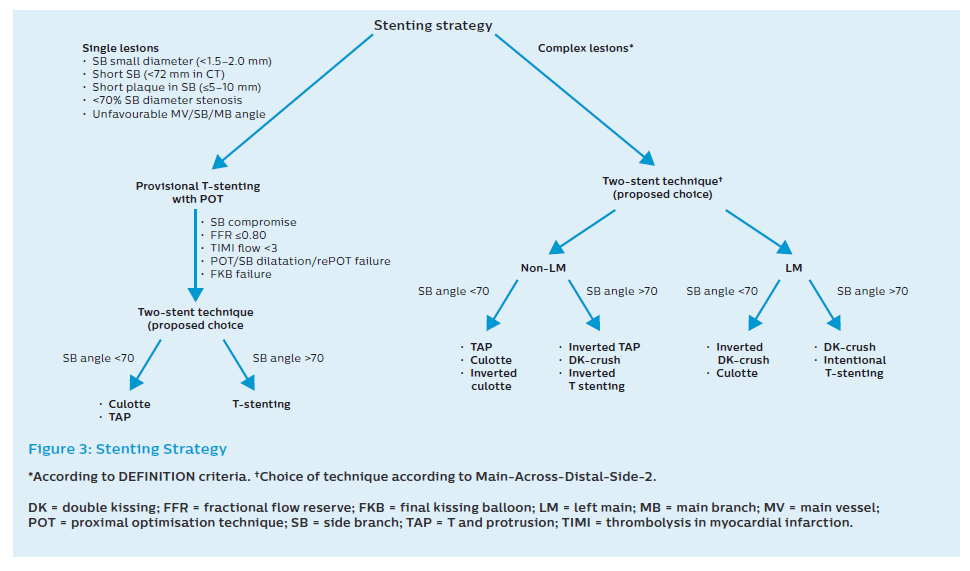
The use of a second stent may be needed in about 10% of cases treated with a provisional approach. The second stent is indicated when the angiographic result is clearly suboptimal (Figure 3).
When flow remains reduced, subsequent SB stenting can be performed using either T, T and protrusion (TAP) or culotte. The choice of T versus TAP can be made by careful imaging with stent enhancement to see whether there is MB stent malapposition on the opposite wall to the carina after KBI and POT. This is more likely after the MB stent is crossed distally, and the POT is accurate. Under these circumstances, T stenting can be considered. In most circumstances, accepting the creation of a small neocarina using the TAP technique is advised.
Both T stenting and TAP should routinely be finalised with a KBI and a POT. Careful placement of the final POT balloon is essential in both techniques, but particular care is needed to avoid the neocarina following the TAP technique (to prevent crushing the protuberant SB stent). Following MB stenting, focal ostial ‘pinching’ with the normal flow in both branches is quite common. Although it may be tempting to deploy a second stent under these circumstances, it is usually unnecessary. Decision-making can be facilitated by pressure wire placement in the SB; in the absence of an ischaemic measurement, a second stent to the SB is clearly unnecessary. When the physiological index suggests borderline ischaemia, management is more controversial but kissing/ostial SB balloon dilation and further careful POT can be considered.9,19–21,33
Elective Two-stent Techniques in Bifurcations
Complex lesions require generally a two-stent strategy. According to the DEFINITION study, lesions were defined as complex when there was ≥70% SB diameter stenosis (≥90% diameter stenosis in non-LM bifurcations) with stenosis length >10 mm and additionally two out of six following minor criteria were met: moderate-to-severe calcification, multiple lesions, LAD–LCx bifurcation angle <45 degrees, MV reference vessel diameter <2.5 mm, thrombus-containing lesions or main vessel lesion length >25 mm.54
Recently, Chiabrando et al. performed a meta-analysis comprising 14 studies and data on 4,285 patients. Double kissing (DK) crush and mini-crush were associated with significant reductions in MACE, TVR, and TLR when compared with the provisional stenting (RR 0.31–0.55 [all p<0.01] and RR 0.42–0.45 [all p<0.02], respectively) and with the remaining bifurcation techniques (RR 0.44–0.55 [all p<0.05] for DK crush and RR 0.37–0.45 [all p<0.05] for mini-crush). In addition, culotte and crush were associated with an increased risk of stent thrombosis compared to provisional stenting (RR 3.25–4.27 [both p<0.05]) and to DK crush (RR 3.02–3.99 [both p<0.05]).55 Nevertheless, Kim et al. showed that in distal LM stenosis there were no significant differences in 1-year target lesion failure between the two strategies (one stent versus two stents) among non-true bifurcation lesions (6.5% versus 4.9%; HR 1.31; 95% CI [0.35–4.88]; p=0.687) and true bifurcation lesions (17.6% versus 21.7%; HR 0.76; 95% CI [0.20–2.83]; p=0.683).56
The elective treatment of bifurcation lesions with complex anatomy and diffuse atherosclerotic involvement of both the MV and the SB is more likely to require a two-stent approach. Two technical issues are critical in these patients. First, lesion preparation before stenting and, second, the procedure must involve KBI followed by final POT. Complete stent expansion facilitates optimal scaffolding and achieves the best acute vessel lumen gain. Malapposed or underexpanded stent struts can affect prognosis by triggering both restenosis and/or stent thrombosis. Accordingly, accurate and extensive postdilatation is required. Elective use of two stents is indicated in very complex lesions with calcified SB and/or ostial disease extending >5 mm from the carina and in bifurcations with a major SB whose access is particularly challenging. Operators have a wide range of theoretical treatment options well summarised by the updated and amended Main-Across-Distal-Side-2 classification, which also included – for the first time – dedicated bifurcation stents.43
All two-stent techniques share the increased risk of stent thrombosis. Although this risk can be reduced by a scrupulous technique, including mandatory kissing inflations and performing final POT, patient adherence to dual antiplatelet therapy is crucial.9,19–21 The advantages and disadvantages of the different techniques are presented in Table 2 and Figure 3.
Evaluation of Post-stent Implantation
Fractional Flow Reserve
As mentioned earlier, the SB ostium’s angiographic appearances after MV–MB stenting have previously been demonstrated to be unreliable, with only a quarter (27%) of cases with a residual angiographic narrowing of ≥75% in the SB being found to have a functionally significant narrowing in pressure wire studies. After bifurcation stenting of the distal LM and non-LM bifurcation, pressure wire assessment of the SB appears to be beneficial if a single cross-over stent technique is used.57
However, the evaluation might be difficult since the pinching could be transient because of vascular wall oedema, minor intramural haematomas and plaque shift, prone to remodelling. One should remember that FFR value in SB >0.80 (or iFR >0.89) before MB stenting does not exclude a subsequent need for SB treating and an FFR value >0.80 (or iFR >0.89) in a jailed SB confirms that SB treatment may be safely postponed.
We also should consider potential FFR/iFR use limitations such as the risk of SB ostium dissection and an increase in the procedural time (with an increase in radiation and contrast volume) when rewiring the SB through the MV–MB stent. When distal LM is considered the risk may, at least in theory, be smaller because of the larger vessel diameters.
Intravascular Ultrasound
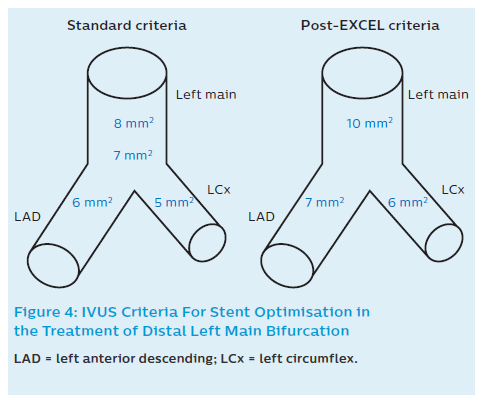
After stent implantation, IVUS can be used to rule out residual edge stenosis or edge dissection (geographic miss), evaluate stent expansion and apposition, rule out accidental abluminal rewiring, assess complications and – in some cases – verify guidewire position after SB re-crossing.9,19–21 Recommendations in non-LM and LM bifurcations include the following considerations: A relative stent expansion of >80%, i.e. minimum stent area (MSA) divided by mean reference lumen area, should be obtained. In non-LM lesions MSA should be >5.5 mm2 by IVUS (and >4.5 mm2 by OCT). In the case of LMs, the desired values are based on the paper by Kang et al.58 They evaluated IVUS predictors of in-stent restenosis (ISR) after LM bifurcation stenting. Post-stenting IVUS-MSA cut-offs that best predicted ISR on a segmental basis were 5.0 mm2 (ostial LCx ISR), 6.3 mm2 (ostial LAD ISR), 7.2 mm2 (ISR within the polygon of confluence [POC, confluence zone of LAD and LCx]), and 8.2 mm2 (ISR within the LM above the POC; Figure 4). A smaller MSA within any of these segments was associated with a greater risk of angiographic ISR and clinical MACE. Therefore, improving underexpansion with these optimal IVUS criteria may reduce cardiac events after DES treatment for unprotected LM disease.58 Based on the EXCEL trial results, it has recently been suggested that for large people, higher post-stenting cut-offs should be applied in order to decrease TLR rates, i.e. 9.8 mm2 (10) in LM, 7.3 mm2 (7) in LAD, and 5.7 mm2 (6) in LCx (Figure 4).59
The clinical significance of acute malapposition is debatable. However, large stent malapposition should be avoided and corrected if anatomically possible. Full apposition may facilitate early strut coverage. Acute malapposition <0.4 mm with longitudinal extension of no more than 1 mm or malapposition does not need to be corrected, since spontaneous neointimal formation is anticipated. Late acquired malapposition is the established factor of late and very late stent thrombosis. Extensive dissections detected by IVUS are independent risk factors of MACE. The following factors increase the risk of events: the presence of residual plaque burden, diffuse lateral (>60°) and longitudinal extension (>2 mm), involvement of deeper layers (medial or adventitia) and localisation distal to the stent. IVUS may detect the stent edge’s haematoma in case of the angiographic appearance of a residual stent edge stenosis.
Optical Coherence Tomography
The following summary of OCT recommendations after stent implantation in coronary bifurcation has been prepared based on Onuma et al.:32
- Following stenting and before kissing the balloon, the SB ostium strut cell should be re-crossed with the guidewire in the most distal cell to reduce the risk of pushing the struts towards the MV.
- Use OCT to assess the position of the re-crossing guidewire and to exclude erroneous abluminal rewiring. When using 3D OCT, re-crossing should be as distal as possible, avoiding the bifurcation carina.
- If rewiring position is deemed sub-optimal, repositioning can be considered.
- The MB is predisposed to more frequent tissue prolapse and the MV is predisposed to more frequent malapposition.
- Malapposition distance of >300 microns and a longitudinal extension of 1 mm or longer may warrant further postdilatation.
- If edge dissection is detected, additional stenting may be performed in instances where the distal edge dissection’s width is ≥200 microns.
- OCT guidance is feasible and may be considered for interventions of non-ostial LM disease as long as the proximal edge of the stent is not occluding the LM ostium.