Carotid artery stenting (CAS) has increasingly assumed an important role in the management of significant carotid artery stenosis, and recent recommendations (by the UK National Institute for Health and Clinical Excellence [NICE] and the American Heart Association [AHA]) suggest that it is a viable alternative to carotid endarterectomy (CEA) for standard-risk populations.1,2 There is a consensus among experts suggesting that embolic protection devices (EPDs) can reduce the risk of stroke during CAS.1–3 These recommendations are supported by an early meta-analysis.4,5
EPDs may be broadly grouped into proximal and distal devices. The former include flow-arrest systems (MoMa, Medtronic/Invatec, Roncadelle, Italy) and flow-reversal systems, to include the Gore® Flow Reversal System, most commonly deployed via the femoral route (WL Gore, Flagstaff, AZ) and flow-reversal systems employed via direct access to the ipsilateral common carotid artery (CCA) (MICHI system, Silk Road Medical, Sunnyvale, CA). Distal systems include filters (of both the perforated polyurethane membrane mesh and nitinol mesh types – these are numerous and are in widespread use), distal occlusive devices, such as the early distal balloon occlusion device (PercuSurge, Medtronic, Minneapolis, MN), the novel in-stent distal occlusive device (TwinOne®, Minvasys, Gennevilliers, France), and the innovative FiberNet®, combining tight filtration (down to 40 microns, which is beyond the reach of the filtering capacity of distal filters) with flow stagnation (Medtronic, Minneapolis, MN). Among the EPDs in clinical use, proximal EPDs have the theoretical advantage of providing embolic protection during all phases of the procedure.5 Furthermore, although there is the important confounding variable of a learning curve (early series, with independent review of neurological outcomes and independent adjudication of these events, are likely to reflect distal filter use whilst more contemporary series reflect the use of proximal systems), some of the lowest stroke and death outcomes for CAS are seen with proximal systems or with those devices that effect a dual protection strategy (FiberNet).
All types of EPD can provide robust embolic protection against macroemboli, which are likely to be associated with clinically relevant stroke. However, if subclinical outcomes are scrutinised (microembolic signal [MES] on transcranial Doppler [TCD] and diffusion-weighted magnetic resonance imaging [DWMRI] new white lesions), proximal systems demonstrate clear superiority within both cohort analyses and small randomised trials that are adequately powered for these endpoints.6–11
Strategies and Treatment Paradigms
How to choose between proximal and distal embolic protection:
- Selection implies that an important differentiation process occurs and can direct clinical decision-making.
- A policy of ‘minimal censorship’ may be appropriate, employing a number of ‘bailout’ mechanisms to support the use of a proximal EPD over distal filter protection.
- The clinical and subclinical data support a strategy of default use of proximal systems.
The rationale for these bold claims is based on both personal experience and the current evidence base.
Proximal systems employ endovascular clamping, resulting in flow arrest or flow reversal; thus the ultimate concern is that of patient intolerance to this type of device. Patient intolerance is largely unpredictable with regard to the patient’s Circle of Willis or the degree of stenosis of the contralateral carotid artery. This is in part due to the fact that the Circle of Willis is imperfectly displayed on pre-procedure gadolinium-enhanced magnetic resonance angiography (MRA), as the posterior communicating arteries are not well delineated with this modality, and computed tomography angiography (CTA), with superior spatial resolution resulting in a more faithful representation of Circle anatomy, is not often performed as a focused review area (the CTA encompassing the extra- and intracranial carotid circulation from arch origin to Circle). Four- or six-vessel selective angiography is perhaps the most sensitive means of evaluating the Circle but it is encumbered by an undoubted additional microembolic burden. Perhaps the most important predictor of patient tolerance is procedural, i.e. ‘on-table’, systolic blood pressure. Juan Parodi, inventor of the original flow-reversal system (ArteriA, now Gore Flow Reversal) suggests that an intra-procedural systolic blood pressure of 160 mmHg or more supports patient tolerance. This is easily achieved; withholding the patient’s usual antihypertensive medication on the morning of the procedure, combined with adequate intravenous (IV) hydration coupled with a degree of patient anxiety (IV conscious sedation should be avoided in order to adequately assess tolerance clinically), will usually suffice. If it does not (a scenario most likely to occur in those patients undergoing CAS pre-cardiac surgery, who are on myriad antianginal and/or heart failure medications, when there is bilateral significant carotid disease), the blood pressure may be manipulated by judicious procedural administration of pressor agents. Note that some units (including our own) will also starve the patient from midnight on the evening pre-procedure; seizure has been reported in the worldwide literature, resulting from ‘intolerance’, although this outcome is rare.
Patient intolerance, no matter how unpleasant to witness for the operating team, is better tolerated by the patient than an embolic shower. As long as the intervention proceeds in a timely, controlled fashion, the neurological symptoms (yawning, unco-ordinated movements of the mouth, failure to comply with commands, drowsiness, obtundation and possibly seizure) will reverse completely without residual deficit. The patient will not remember these events and the operating team need to develop ‘tolerance of intolerance’. Many surgeons choose to perform selective shunting with the surgical clamps on during CEA, or do not shunt at all, even when the procedure is performed under local anaesthetic, when there is clear evidence of intolerance. It should be noted that the surgical clamp time will often exceed endovascular clamp times; the median unshunted clamp time in the North American symptomatic carotid endarterectomy trial (NASCET) was 31 minutes.12
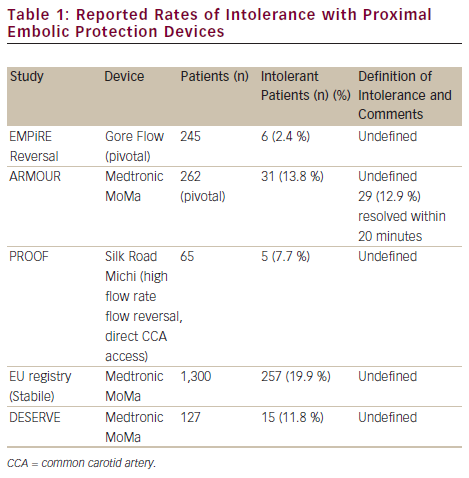
One important consideration is that intolerance remains poorly defined in the endovascular literature and may be largely subjective, a prime example being the Silk Road medical embolic protection system: first-in-man (PROOF) analysis of the Michi system employing flow reversal via a mini-cervical cut-down. This is an on-going analysis and outcomes from the first 44 patients have been reported (see Table 1).11 To date, 65 patients have been treated. Intolerance was reported in six out of seven cases in the 65-patient cohort early in the Düsseldorf experience and only once towards the end of this group’s experience.
Bailout
There are a number of ways to manage intolerance. Firstly, it should be recognised that the majority of cases manifest intolerance (if at all) at two specific time points, of which one is early in the procedure, in which case, unclamping the CCA and allowing antegrade flow for a few minutes before reinflating the balloon in the CCA may suffice, through some poorly understood ‘conditioning’ mechanism. Often one will notice compensatory hypertension during endovascular clamping in tolerant patients. After stent placement and/or post-dilatation, intolerance may manifest because the carotid baroreceptors and compensatory mechanisms for blood pressure control have been overcome. At this point, the procedure is close to completion and it is appropriate to proceed in a timely fashion.
Alternatively, intermittent clamping can be employed or, once the lesion has been crossed on flow arrest/flow reversal, the operator might wish to resort to distal filter protection. The Michi system, which effects flow reversal via direct CCA access through a mini cut-down, affords additional bailout options: the hand-held flow controller positioned between the arterial and venous ends of the flow-reversal circuit provides ‘no flow’, ‘low flow’ and ‘no flow’ settings and one can switch from ‘high flow’ to ‘low flow’ or ‘no flow’ modes to enhance tolerance, and lastly, as this system is placed via a mini-surgical cut-down (a 2 cm transverse incision just above the clavicle between the heads of the sternomastoid), the operator may opt to convert to conventional CEA.
Relative Contraindications to Proximal Systems
The MoMa and Gore Flow Reversal systems have working sheaths that are integral to the protection mechanism and these are both 9 F, making them more suitable for access via the femoral rather than the radial or brachial arteries. Thus access is an important consideration for these devices; common femoral disease or complex arch anatomy, i.e. bovine or type III, especially with the additional feature of an occluded or significantly diseased ipsilateral external carotid artery (ECA), renders use of these devices more challenging.
Clinical Outcomes – Contemporary
The American multicentre registries Embolic protection with reverse flow study of the Gore Flow Reversal System in carotid stenting of subjects at high risk for carotid endarterectomy (EMPiRE), Proximal protection with the MoMa device during carotid stenting (ARMOUR) and FiberNet embolic protection system in carotid artery stenting trial (EPIC) evaluated the Gore Flow Reversal System, the MoMa and the FiberNet, respectively, while the two-centre German PROOF trial evaluated the Michi system and all mandated independent neurological review of outcomes with independent adjudication of outcome events.13–15 A French-based multicentre cohort investigated the TwinOne system.16 While a variety of stents could be used, each registry focused on a single EPD.
Distal Embolic Protection Systems
TwinOne
Two hundred and nine patients (32.5 % symptomatic) were recruited into this cohort analysis, comprising 217 CAS procedures in a French multicentre study.16 The primary endpoint was all stroke/ death/transient ischaemic attack (TIA); it is not clear if the outcome events were independently audited. The primary outcome occurred in 2.76 % (1.80 % disabling stroke and 0.92 % TIA). There were no significant differences in outcome for symptomatic and asymptomatic populations. The authors comment that inflation of the distal occlusion balloon within the leading end of the stent prevents spasm in the distal internal carotid artery (ICA) and the feared, but rare, complication of distal ICA dissection. Furthermore, the authors noted a reduced mean occlusion time with the TwinOne as compared with previous version of the distal balloon occlusion device: 3 minutes, 9 seconds (range 1–13 minutes). However, the mean procedure time was 51 minutes (standard deviation 25 minutes).
Filter Devices
The FiberNet is a hybrid filter and distal occlusive device which was evaluated in 237 patients considered to be at high risk for CEA (20 % symptomatic and 21 % octogenarians) in the EPIC trial.15 The primary endpoint was the all stroke/death/myocardial infarction (MI) rate at 30 days and the study employed a US Food and Drug Administration (FDA)-approved objective performance goal of 8.3 % derived from the Acculink for revascularization of carotids in high-risk patients (ARCHER) III results. The composite endpoint occurred in 3 % of the full population, in 4.2 % of symptomatic patients (n=48) and in 8.2 % of octogenarians (n=50), the stroke rate being 6.3 % in this subset.
Proximal Embolic Protection Systems
The ARMOUR trial evaluated the MoMa device in 262 patients considered to be at high risk for CEA on the basis of various anatomical and clinical parameters (15 % were symptomatic and 28.9 % were ≥80 years of age).14 The primary endpoint was the 30-day all stroke/MI/death rate which was compared with a performance goal derived from previous trials evaluating distal EPDs in which the averaged point estimate event rate was 13 %. The primary outcome by intention-to-treat analysis for the full population was 2.7 %. In the symptomatic group there were no stroke/death events and the all stroke/death/MI rate was 3.1 % in patients ≥80 years of age.
Diffusion weighted mri evaluation of the effetiveness of endovascular clamping during carotid artery stenting with the MoMa deVicE (DESERVE) study was a single-arm prospective multicentre EU study evaluating the MoMa device in 127 subjects (a mix of standard and high-risk patients). The primary endpoint was the 30-day rate of stroke/death/MI. A total of 12.6 % were symptomatic. The mean clamp time was 4.46 ± 2.31 minutes. The primary endpoint occurred in three out of 127 patients (2.4 %).9 The DWMRI analysis performed within this study is presented below.
An EU multicentre analysis of 1,300 patients undergoing MoMa-protected CAS with independent review of neurological outcomes in a mixed high/standard-risk population was recently reported.17 A total of 27.8 % were symptomatic. The primary outcome, 30-day stroke/death, occurred in 1.38 % of patients (3.04 % for symptomatic and 0.82 % for asymptomatic patients, p<0.05).
The Gore Flow Reversal System was evaluated in the EMPiRE trial and included 245 patients considered to be at high risk for surgery.13 Thirty-two per cent were symptomatic and 15 % were ≥80 years of age. The primary outcome was the rate of all stroke/death/MI and TIA at 30 days. The all stroke/MI/death rate was 4.5 %, significantly lower (p=0.002) than with the 11.8 % preset objective performance criterion (with a non-inferiority margin of 5.45 %), calculated as the sum of the weighted averages of 30-day stroke/death/MI rates in seven earlier registries in which a distal EPD was used. The all stroke/death rate at 30 days was 2.9 % (2.6 % in symptomatic patients and 2.6 % in octogenarians), meeting the AHA guidelines for CEA in predefined subsets (i.e. non-octogenarians). Furthermore, the endpoints for non-represented populations within the guidelines (the elderly) were within the acceptable AHA thresholds set for younger patients.
A two-centre German registry, the PROOF study, has evaluated the Michi system. The primary endpoint was major stroke/death/MI at 30 days and outcomes were assessed by an independent neuro-anaesthesiologist. The outcomes in 44 patients (9 % symptomatic) have been reported.11 The major stroke/death/MI rate was zero. One minor contralateral stroke was reported at 30 days in a patient who was clinically well post-stenting and free of lesions on post-procedural DWMRI scans at 30 days. This minor stroke was adjudicated to be unrelated to the procedure or the device by the independent clinical events committee. Transient intolerance to reverse flow was reported in 9 % of cases, but in all cases a stent was successfully placed and the intolerance was managed by minimising the duration of reverse flow during the procedure. There were no cranial nerve injuries in the recent publication, although the study is on-going: it has recruited 65 patients to date and, latterly, a cranial nerve injury (yet to be adjudicated) was reported.
Clinical Outcomes – Historical
Registry data from the US, with independent review of outcome events dating from the years 2000 to 2008, highlight the stroke and death rates from a variety of studies that sought to evaluate a single manufacturer’s stent and distal filter device (Registry study to evaluate the Emboshield bare wire cerebral protection system and Xact stent in patients at high risk for carotid endarterectomy [SECURITY], ARCHER II, ARCHER III, Carotid revascularization with ev3 arterial technology evolution [CREATE] I, CREATE II, Medtronic self-expanding carotid stent system with distal protection in the treatment of carotid artery stenosis [MAVERIC] and Carotid artery revascularization using the Boston Scientific EPI filter wire EX/EZ and the EndoTex NexStent [CABERNET]). The randomised trials Stenting and angioplasty with protection in patients at high risk for endarterectomy (SAPPHIRE) and Carotid revascularization endarterectomy versus stent trial (CREST) also provided outcome data during this timeline. All but CREST recruited patients considered to be at high risk for surgery and the vast majority of patients were asymptomatic. Whilst there was clear evidence of improved outcomes over time, the stroke and death rates ranged from 3.5 to 6.8 % and were, in the main, higher than the stroke and death outcomes from the more contemporary ARMOUR, DESERVE, EMPiRE, EPIC and PROOF studies.9,11,13–15
Subclinical Outcomes
The International carotid stenting study (ICSS) was a one-to-one randomised comparison of CAS versus CEA in standard-risk patients in a UK-based international trial.18 In a sub-study of the ICSS trial, 231 patients had pre-procedural and one to three days and one month post-procedural DWMRI scans to compare the microembolic penalty following CEA to that for CAS.19 The new white lesion rate was 50 % for CAS and 17 % for CEA. There were fewer, larger lesions following CEA and many more, smaller lesions for CAS. Five per cent of lesions were in the territory contralateral to the carotid artery being treated in CAS patients, compared with 1 % of contralateral lesions following CEA. In 33 % of lesions detected on post-procedure DWMRI in CAS patients, the lesions were evident on fluid attenuation inversion recovery (FLAIR) imaging at 30 days, implying some level of permanent injury, compared with 8 % for CEA patients. Accepting this, an analysis of cognitive function as a further subset analysis of the ICSS data set revealed no difference in neuropsychometry between treatment limbs, despite an excess of DWMRI lesions following CAS.20
Within the DWMRI sub-study, five centres used filter-type EPDs in the majority and two centres did not routinely employ EPDs. The new white lesion rate was 73 % for filter protection and 34 % for unprotected CAS (p=0.019).
Two small randomised trials have sought to compare control of the microembolic burden with the MoMa device and with filter protection. The first (recruiting 53 patients with lipid-rich plaque as assessed on CTA) demonstrated significantly more MESs with filters than with the MoMa (101 ± 53 versus 22.5 ± 19) and substantially more DWMRI new lesions with filters. The difference in this endpoint did not reach statistical significance, as the study was underpowered for the diffusion-weighted imaging surrogate (n=45).7 The latest trial recruited 62 patients and demonstrated an 87.1 % new white lesion rate following filter-protected CAS and a 45.2 % new white lesion rate following MoMa–protected CAS. This differential in favour of proximal protection was maintained regardless of either symptom or octogenarian status.6
Prospective cohort analyses show comparable and consistent discrepancies between proximal and distal filter devices, in favour of proximal devices. Schmidt et al., in a prospective analysis of 21 patients undergoing MoMa-protected CAS and 21 undergoing filter-protected CAS, demonstrated a total MES count of 57 ± 41 for the MoMa device and 196 ± 84 for the filter (p<0.0001). Sheath and/or protection device placement and retrieval were noted to be universally emboligenic stages of the procedure.8
In the DESERVE study evaluating the MoMa in a multi-site EU registry of 127 patients, the new white lesion rate was 30 %.9
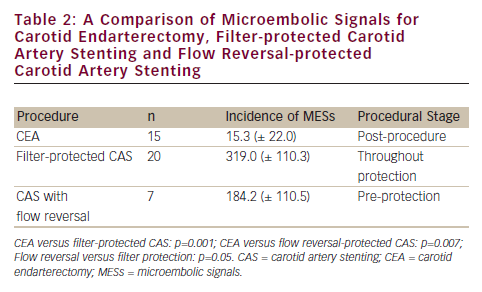
A small, but elegant, non-randomised study compared CEA with filter-protected and flow reversal–protected CAS via a femoral route in 42 patients (see Table 2).10
The stage of vulnerability of the brain with different carotid interventional strategies requires special mention. Flow reversal is associated with significantly fewer MESs than filter protection but the brain is vulnerable pre-protection with flow reversal, implying the embolic penalty posed by catheterisation of the arch and great vessels. If one addresses the important limitation of carotid stenting via the femoral route, CAS might start to approximate the excellent control of microemboli afforded by CEA.
In the PROOF analysis of the Michi system (high flow rate reversed flow via a mini-incision in the ipsilateral CCA, thereby avoiding catheterisation of the arch and great vessel origins), the DWMRI new white lesion rate was 17 %, commensurate with the new white lesion rate for CEA within the ICSS sub-study, and the first time that such tight control of the microembolic burden of CAS has ever been demonstrated.19
Discussion
Despite the mixed results of the early trials, it is clear that there has been a stepwise improvement in CAS stroke/death outcomes with time. This is partly due to learning curve issues, i.e. better understanding of both patient- and lesion-related factors along with an enhanced appreciation of variable procedural risks associated with different interventional strategies, and partly due to improvements in protection strategy offered by newer EPDs, most often employing proximal protection or dual tight filtration/distal occlusion (GoreFlow Reversal, MoMa, Michi, FiberNet).
It is clear from small randomised trials and cohort analyses that proximal embolic protection is superior to distal filter protection in terms of the control of microemboli. The prolific new white lesion rate and innumerable MESs following filter-protected CAS require elucidation. Filters afford a ‘controlled embolisation’. Undoubtedly, some of the MES events are due to gaseous elements trapped in the material of the filter but they will, to some extent, translate into new white lesions on DWMRI. Filter-through, ‘peri-flow’ and fragmentation of larger particles may in some way explain the findings. It should also be noted that the fate and clinical relevance of these lesions is far from clear.21 The latest analysis of cognitive function between those patients with prolific new white lesions (filter-protected CAS) and those with far fewer lesions (CEA) does not provide compelling evidence of their significance.20 Further work is required.
The difference in new white lesion rates for proximal protection with a single device, MoMa, (i.e. 45 % in the Prospective, randomized trial of proximal balloon occlusion versus distal filter embolic protection in patients undergoing carotid stenting (PROFI) trial and 30 % in the DESERVE study), again requires explanation. The patient populations in these two analyses were comparable. Differences in procedural protocols may be a relevant consideration: requirements for on-table four-vessel selective angiography and mandatory contrast injection once the standing column in the ICA had been achieved in the flow-arrest situation (thus partly negating protection) may contribute to differences in outcome. Avoiding the arch and great vessel origins may further improve the microembolic profile of CAS but this currently requires a hybrid CAS/CEA procedure with the Michi system. Percutaneous direct CCA access systems are awaited.
Conclusions
The current evidence case suggests improved stroke and death outcomes in contemporary series evaluating proximal EPDs compared with earlier series employing filter-type protection. The learning curve, constituting an important confounder that cannot easily be corrected for, must, however, be acknowledged. Accepting this, a clear and consistent message is delivered by a number of small randomised trials and cohort analyses, i.e. that proximal systems are superior to filters in control of the microembolic profile of CAS (both MESs on TCD and new white lesions on DWMRI). The clinical and subclinical data therefore support a strategy of default use of proximal systems, employing bailout mechanisms on those infrequent occasions when intolerance demands management. The arch represents hostile territory for carotid intervention via the femoral approach and attention is turning to direct carotid access with flow reversal.