Brugada syndrome (BrS) remains one of the most common inherited channelopathies associated with an increased risk of sudden cardiac death (SCD), with a worldwide prevalence of approximately 0.05 %.1–3 It is accepted that appropriate utilisation of the ICD in high-risk patients with aborted SCD and haemodynamically compromising arrhythmias is life-saving. However, there remains a lack of consensus on the management of patients with BrS and no history of ventricular arrhythmias or aborted SCD, especially in the context of a resting type 1 coved ECG pattern. The current guidelines and consensus statement recommend ICD implantation in patients with BrS with spontaneous type 1 ECG pattern and probable arrhythmia-related syncope, the latter being heavily dependent on the quality of the syncope history.4,5 This recommendation is based on several studies that demonstrated a higher risk of arrhythmic events in such patients compared to those without these factors present.2,3,6,7 However, whether other clinical factors are better predictors or facilitate more refined risk stratification before any arrhythmic event is still up for debate. This is especially important as the first clinical event may be cardiac arrest. Indeed, the recent Survey on Arrhythmic Events in BrS (SABRUS) study, which specifically evaluated patients presenting with a lethal arrhythmic event, found that 25 % of patients did not reach the current ICD implantation criteria.8
Brugada ECG Pattern or BrS
Before refining risk stratification strategies, it is important to clarify what establishes a diagnosis of BrS. The guideline defines it as the presence of a type 1 Brugada ECG pattern, whether drug-induced or spontaneous.4,5 However, others argue that, without the presence of symptoms, the ECG features only indicate the presence of a Brugada pattern ECG and not the syndrome itself. This argument stems from the fact that the yearly cardiac event rate is only 0.5 % in these patients, compared with 1.9 % in patients with a history of syncope.3 With the annual risk of death from any cause being around 0.4 % in the middle-aged male population most commonly affected,9 the additional risk of BrS-induced cardiac arrest appears minimal in the asymptomatic population.10 Therefore, it can be argued that labelling patients with only a type 1 Brugada ECG pattern and no symptoms as having a syndrome, and proposing that they are at a significantly enhanced risk of SCD, might be inappropriate. Offering advice on the aggressive treatment of a fever, avoidance of type 1 ECG pattern-provoking drugs and offering review in the presence of symptoms may be sufficient for this cohort of patients. This is supported by the up-to-date guideline, which provides a class I recommendation for observation without therapy in these patients.4
However, there is a spectrum of risk. Sacher et al. showed that 12 % of BrS patients who were asymptomatic at ICD implantation had appropriate ICD therapy during a 10-year follow-up period.10 Furthermore, the presence of a spontaneous type 1 ECG pattern alone has been shown to be associated with a lower cumulative survival,2 a doubled risk of arrhythmic events3,11,12 and shorter time to first arrhythmic event3 compared with a drug-induced type 1 ECG pattern. Therefore, the diagnosis of an isolated Brugada ECG pattern should potentially be restricted to those patients with a drug-induced type 1 ECG pattern and exclude those with a spontaneous type 1 ECG pattern. Furthermore, as the presence of both a spontaneous type 1 ECG pattern and syncope is associated with a significantly higher risk of cardiac arrest compared with a spontaneous type 1 ECG pattern alone, those with the former should potentially be labelled as a high-risk group and those with the latter as an intermediate-risk group.3
Ajmaline Testing
Another area that requires clarification is the use of ajmaline testing. Ajmaline is used to provoke a type 1 Brugada ECG pattern. Along the same lines as already discussed, the presence of a provoked type 1 ECG pattern in the absence of symptoms is not associated with a significant risk of SCD: 0.3 % over 3 years.3 This raises the question whether performing this investigation is appropriate if it will not result in a change in patient management yet might lead not only to enhanced patient anxiety but also to unnecessary risk associated with ajmaline testing. This is of particular importance as studies have reported high rates of concealed type 1 Brugada ECG pattern in asymptomatic patients;13 should all of these patients be labelled with a syndrome that has life-long implications?
However, if patients are symptomatic, ajmaline testing is warranted because of the increased risk of SCD seen in these patients with BrS.10 Further to this, in those with a family history of SCD in first-degree relatives, ajmaline testing can not only help to explain the cause of death in the proband but also, if positive, to identify family members with potential high-risk features.5
Risk Stratification in BrS
Identifying factors that are associated with an increased risk of ventricular arrhythmias and SCD in BrS is a significant challenge. With ICDs being associated with a life-long complication risk of up to 45 %,14 the decision to implant these devices should not be taken lightly. Indeed, although the advent of subcutaneous ICDs could reduce the risk of transvenous lead problems in the long term, there remains the morbidity associated with inappropriate device therapies and the risk of infection with multiple generator changes over time.
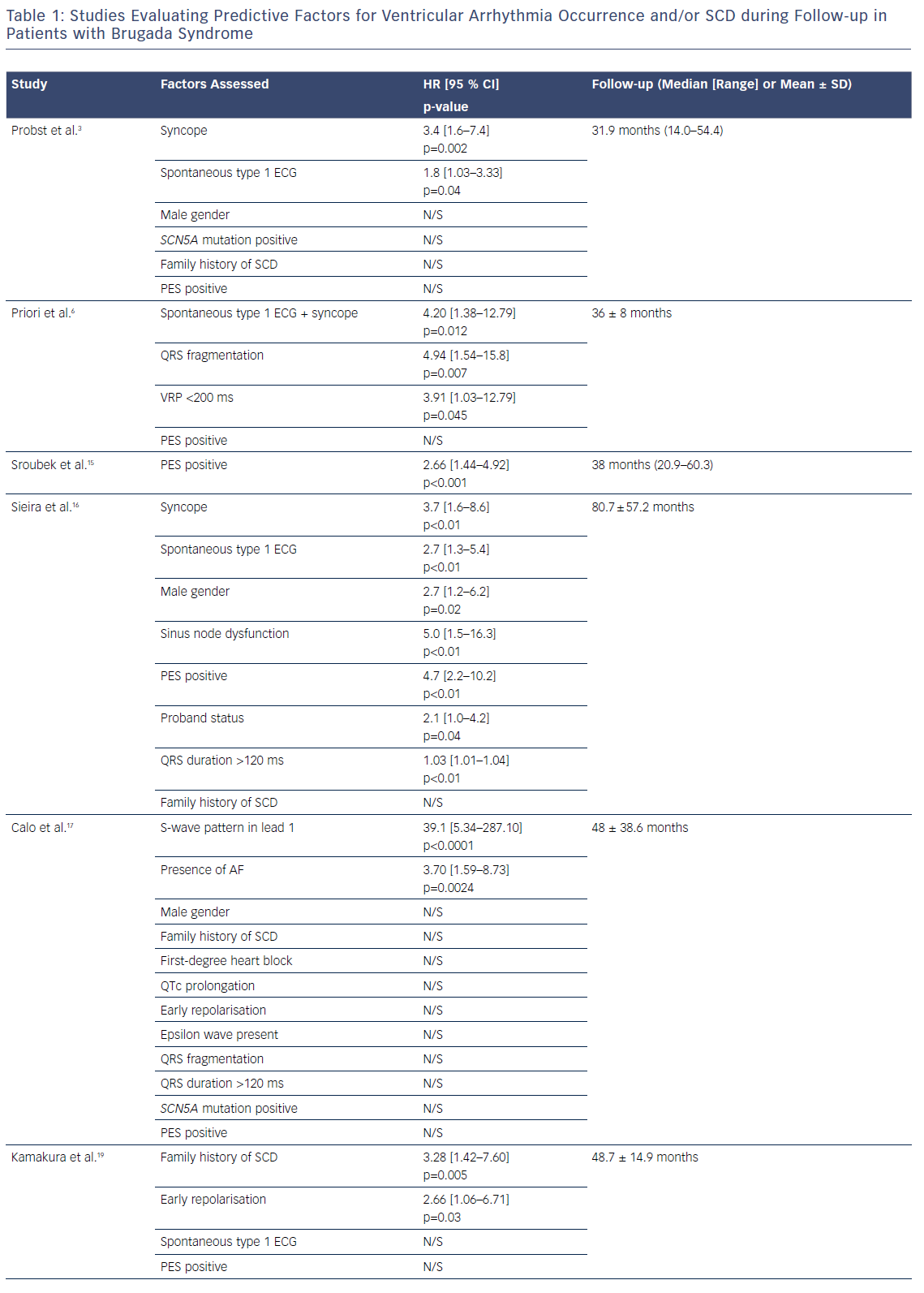
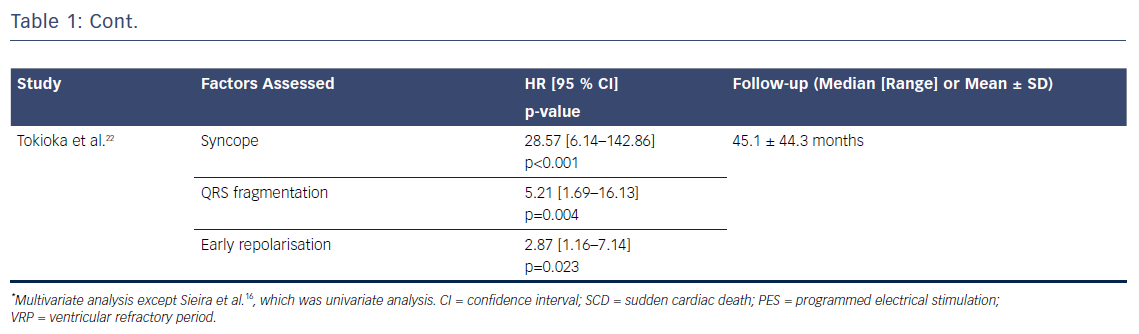
Several risk factors have been proposed over the years. The France, Italy, Netherlands, Germany (FINGER) registry, the largest international cohort to date, assessed the role of six proposed risk factors in predicting ventricular arrhythmic events: syncope, spontaneous type 1 ECG, gender, family history of SCD, inducibility of ventricular tachyarrhythmias during electrophysiological study and presence of an SCN5A mutation.3 Syncope and spontaneous type 1 ECG pattern were the only significant predictors. These factors are the only ones that have remained consistent in their predictive role in other studies.3,6,15,16 Other markers, however, either yield conflicting data or have only been assessed in a small proportion of studies, making it difficult to evaluate their true role in the risk stratification of Brugada patients (Table 1).
Factors with Conflicting Evidence
A positive programmed electrical stimulation test is a good example of the factors in this pool of conflicting evidence, in that it has been shown to be a strong predictor of ventricular arrhythmias in BrS in some studies15,16 while in others it has played no role in BrS risk stratification.3,6,17,18 Recent data from the FINGER registry suggest that a positive study with up to two extra stimuli could have prognostic significance, and a negative study has a high negative predictive value.15 The presence of an SCN5A mutation3,6,15,17 and family history of SCD3,15,17 are further factors whose role in risk stratification of Brugada patients remains uncertain. Based on these findings, utilising programmed electrical stimulation and genetic mutation testing in the risk stratification of these patients would not be strongly recommended on a population level unless there is a particularly malignant family history or specific highly arrhythmogenic mutations.
ECG Markers with Promising Predictive Value
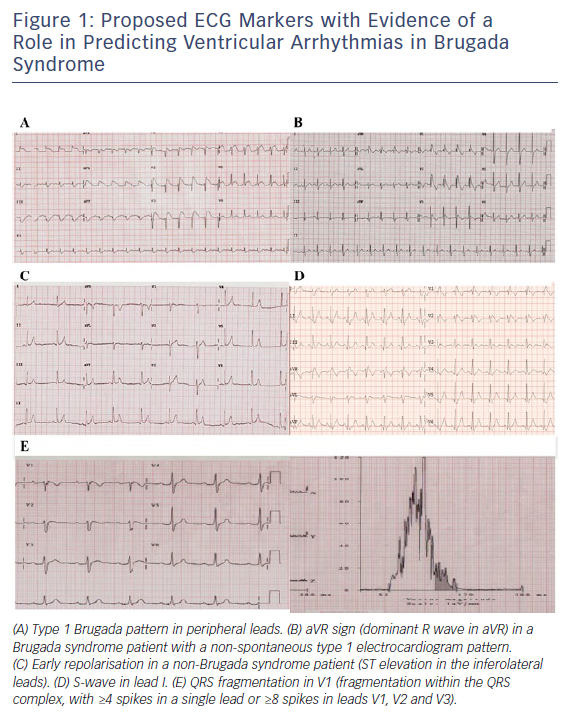
The presence of a type 1 Brugada pattern in peripheral leads,18 early repolarisation (ER),19–22 aVR sign23 and S-wave in lead 1,17 and fragmented QRS24 (Figure 1) have been associated with an increased risk of ventricular arrhythmia occurrence during follow-up. However, as these factors have not been consistently assessed in a range of studies, it is unclear whether their predictive value applies across a general BrS population. The presence of ER has already been associated with a higher risk of ventricular arrhythmic events in patients with idiopathic ventricular fibrillation,25,26 and it is therefore possible that its presence indicates an arrhythmogenic predisposition. It is plausible that the presence of type 1 Brugada pattern in peripheral leads is indicative of a higher Brugada substrate burden and, as a result, may be associated with a greater risk of ventricular arrhythmia. Evaluating all these factors together in a large BrS population is required to effectively establish their importance.
Risk Scoring Model in BrS
A number of studies have combined risk factors to predict the risk of SCD.11,16,27 The initial study by Delise et al. showed that no single risk factor was able to identify BrS patients at high risk of arrhythmic events and that a multi-parametric approach was a more robust strategy.11 The authors showed that the subjects at highest risk were those with a spontaneous type 1 ECG pattern and at least two further risk factors (including syncope, family history of SCD and positive programmed electrical stimulation). More recently, Sieira et al. evaluated several factors and proposed a score that included the presence of: spontaneous type 1 ECG pattern; early familial SCD (<35 years old); positive programmed electrical stimulation; presentation as syncope or as aborted SCD; and sinus node dysfunction.16 The authors demonstrated a predictive performance of 0.82 for this score. They showed that a score greater than two conferred a 5-year event probability of 9.2 %. However, it is important to consider several points prior to implementing the use of this score. The factors utilised in this risk score were derived only from univariate analysis. Since no multivariate analysis was conducted, it is unclear whether all these factors have an independent predictive role for ventricular events. Furthermore, the validation of the risk score that established its predictive performance was undertaken in a cohort from the same centre. Since the risk score has not yet been evaluated externally and the baseline characteristics of the cohort showed several differences to those of other, larger studies, it is unclear whether this predictive performance is applicable to the general BrS population. Therefore, even though this approach of integrating risk factors is promising, further validation in other BrS cohorts is warranted prior to its use in clinical practise. However, there is clearly a role for combined risk factor scoring in BrS.
The Future in BrS
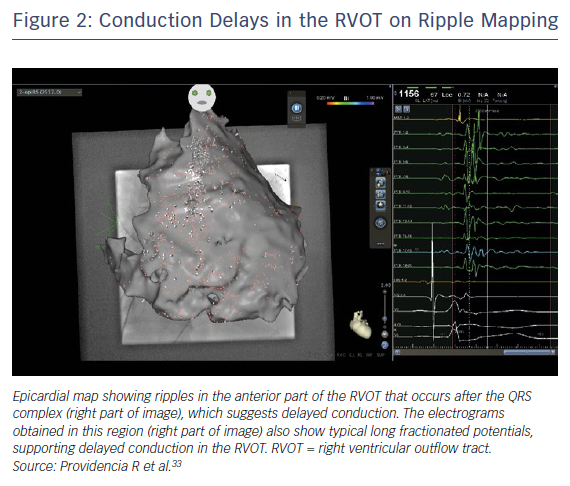
Several studies have demonstrated prolonged right ventricular outflow tract (RVOT) activation with marked regional conduction delay and fractionated late potentials in patients with BrS.17,24,28 As well as utilising clinically derived risk factors in risk stratification, there may be a role for more refined evaluation of the arrhythmogenic substrate. Electrocardiographic imaging (ECG-I) has demonstrated marked conduction delays in the RVOT (Figure 2), and this area of delay is expanded in the presence of ajmaline.29 The degree and/or area of delay may be another useful biomarker to predict risk; indeed, an ECG-I approach to risk has been proposed in a preliminary study utilising exercise stress.30 Although genetic factors are important, their role to date has been limited to individual mutations; the burden of specific variants may also be utilised in the future to refine risk scoring.
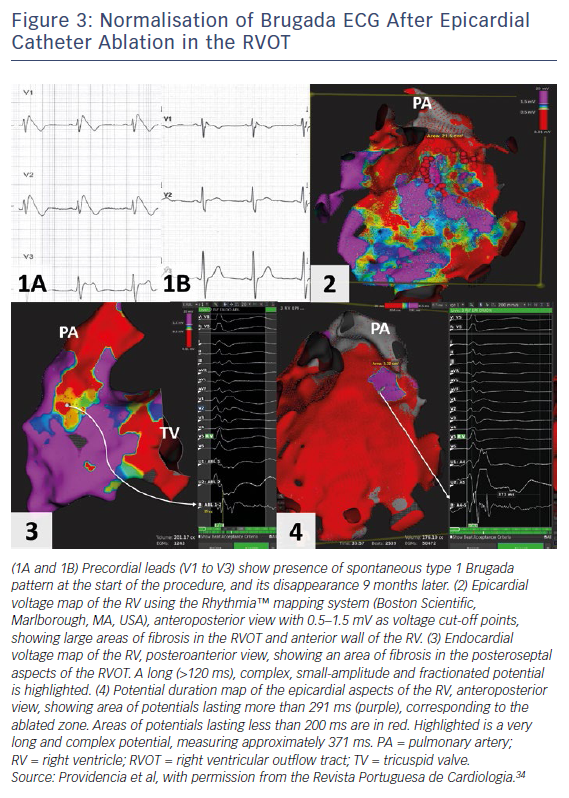
The current American College of Cardiology, American Heart Association and Heart Rhythm Society guideline for management of ventricular arrhythmias recommends catheter ablation or quinidine for patients: experiencing recurring shocks for ventricular arrhythmias; and with spontaneous type 1 pattern and symptomatic ventricular arrhythmias who either are not candidates for an ICD or decline an ICD (class I recommendation, level of evidence B [non-randomised for both]).4 Two studies have shown that epicardial catheter ablation performed in the RVOT, with a view of eliminating this arrhythmogenic electrophysiological substrate, resulted in the normalisation of the Brugada ECG in majority of patients, even after ajmaline (Figure 3).31,32 In the study with the larger cohort of patients,31 the current follow-up is less than 1 year; therefore, a longer follow-up period is required to ensure that the ablation effect is permanent. Indeed, a clinical trial is now in progress to evaluate the role of prophylactic ablation in BrS (ClinicalTrials.gov identifier: NCT02641431). However, given that the condition may be a progressive disease, issues relating to risk stratification even after ablation will remain for the foreseeable future.