In the context of an ageing population, care for valvular disease is becoming an increasingly important field. A recent large-scale population-based study found the prevalence of valvular disease to be 8.5 % in the 65–74-year-old group and 13.2 % in those over 75 years. 1 While mechanical surgical valves are more durable, bioprosthetic valves are often used as they are less thrombogenic and do not require life-long anticoagulation. A recent meta-analysis found that bioprosthetic valves have significantly fewer thromboembolic events and anticoagulation-related events, including major bleeding. 2 A large multi-centre database study also found that from 1997 to 2006 the use of bioprosthetic valves increased from 40 % to 80 %. 3 This trend illustrates the importance of managing the long-term dysfunction events of bioprosthetic valves, as they tend to degenerate within 10–20 years, requiring more re-operation than mechanical valves. 2,4
Traditionally, patients with degenerated bioprosthetic valves required re-operation; however, these patients are often of advanced age and have other co-morbidities that put them at high risk for surgical complications. Transcatheter valve-in-valve (VIV) implantation has become a potential alternative procedure for high-risk patients with degenerated bioprosthetic valves, and the Valve-in-Valve International Data (VIVID) Registry has been established to collect information on these procedures. A recent meta-analysis of degenerated aortic valves found that VIV implantation achieved comparable haemodynamic outcomes to open aortic valve replacement while at a lower risk of stroke and bleeding. 5 In addition, VIV achieved a comparable perioperative mortality rate to the open procedure, with reported 83 % survival after 1 year. 5,6 While there have been reports of success during intermediate term follow-up, 7,8 the long-term durability of VIV implants is unknown. The three main adverse events of VIV are device malpositioning (15 % of VIV patients in the VIVID Registry, mainly in stentless surgical valves), coronary obstruction (~3 %), and elevated (defined as >20 mmHg) residual transvalvular gradient (28.4 %). 9 With greater operator experience, the rate of both malpositioning and coronary obstruction seems to decrease, while residual elevated gradients remains an issue. 10
Elevated post-procedural gradients can be partially explained by the non-distensible nature of bioprosthetic valves, often resulting in an underexpansion of VIV implants. 11 The underexpansion of the transcatheter heart valve (THV) has been shown in vitro to create localised high-stress regions within the leaflet commissures or within the belly of the leaflets. 12 This increased mechanical stress and deformation has been hypothesised to result in accelerated degeneration of these valves. In addition, underexpansion results in a higher gradient after VIV procedure (average mean gradient of 15.8 ±8.9 mmHg reported in the VIVID Registry) as compared to regular transcatheter valve implants in native aortic valves (5–10 mmHg), therefore creating unique challenges and considerations for the VIV procedure. 6,13 These haemodynamic considerations are critical because studies have shown that elevated post-procedural mean gradient is associated with worse clinical outcomes. 14
In addition, an elevated post-procedural mean gradient is inherently present with prosthesis–patient mismatch (PPM), defined as having too small of an effective orifice area in relation to the patient’s body size. 15 PPM has been shown to be associated with reduced recovery of ventricular hypertrophy, increased cardiac events and mortality. 15 A large-scale meta-analysis of 27,000 surgical patients found that PPM is associated with both an increase in all-cause mortality and cardiacrelated mortality. 16 Due to the nature of the VIV procedure, the rate of PPM post-VIV has also been reported to be higher than surgical valve replacement. 17,18 Post-procedural mean gradient and its associated PPM is influenced by a combination of factors, including surgical valve size and its aetiology of degradation, as well as the THV device type, size and depth of implantation. This review will summarise the newest findings in this area and explore modifiable factors that could improve the haemodynamic outcomes of a VIV procedure.
Pre-surgical Considerations
The VIVID Registry revealed that patients with smaller (manufacturer label size ≤21 mm) and intermediate bioprostheses (manufacturer label size >21 and <25 mm) as compared to larger bioprostheses (manufacturer label size ≥25 mm) had a worse survival rate. 10 This difference in mortality may be explained by the increased restriction on THV expansion in smaller surgical valves, which can lead to an increased likelihood of developing elevated post-procedural gradients, PPM and less improvement in clinical functional status. Potentially, patients with smaller surgical valves are at a greater risk of having PPM prior to the VIV procedure, leading to a situation that will not be solved by VIV alone, without removing the small surgical valve during repeat surgery. Data from the VIVID Registry suggests that patients who already had severe PPM of their surgical valve had significantly worse outcomes after VIV. Having severe PPM of the original surgical valve was found to be a strong correlate for 1-year survival, with severe PPM of the surgical valve being associated with 71.8 % 1-year post-VIV survival as opposed to 85.8 % survival in the combined no PPM and moderate PPM group (unpublished data from the VIVID Registry). This suggests that patients with severe PPM may not be optimal candidates for an aortic VIV procedure and that this factor should be carefully evaluated in candidates for VIV procedures.
In addition to the surgical valve size, the type of degeneration is also useful to consider, as a predominant surgical valve stenosis has been associated with increased mortality when compared to patients with regurgitation. 10 This may also be related to the haemodynamic outcomes, as a stenotic bioprosthetic valve would impede the expansion of the THV device more than a regurgitant one. This restriction increases the likelihood of developing elevated post-procedural gradients, which could have contributed to the worse outcomes seen in the study.
One important distinction to make is that, for bioprosthetic surgical valves, the manufacturer’s label size matches the outer diameter of the device; but the truly relevant measurement for VIV sizing is the internal diameter, as this is where the THV will actually be expanded. This value may vary from 1 to 4 mm from the reported label size, depending on the manufacturer. 11 The true internal diameter is currently available in the “VIV Aortic” app from Mr Vinayak Bapat (UBQO Limited) and should be used by operators to enable appropriate THV valve size selection and also to predict the risk of poor post-procedural haemodynamics.
As the risk of surgical valve degeneration requiring reoperation is high, new surgical valves that are appropriately designed to provide better conditions for VIV are urgently needed. Proposed features for these new surgical valves could include clear fluoroscopic markings indicating size and the region of appropriate positioning, more flexible and expandable annuli to allow for complete expansion, and larger internal diameters. 19 These new features would facilitate the VIV procedure and help to reduce potential complications such as THV misplacement, while also allowing for better haemodynamics.
If possible, it would be preferable to select the surgical valve with the largest effective orifice area, in order to facilitate optimal future VIV procedures. Some studies have reported that haemodynamic outcomes are better in supra-annular positioning compared to intraannular bioprostheses. 20,21 Recently, sutureless valves have also been proposed as an alternative to aortic root enlargement; they have an acceptable safety profile while allowing for a larger valve size to be inserted. 22 Another meta-analysis showed that stentless valves provide larger effective orifice areas, lower mean gradient and greater ventricular mass regression. 23 In addition, techniques such as aortic root enlargement to allow for the insertion of larger bioprosthetic valves are occasionally considered for patients at risk of severe PPM. This procedure prolongs the cardiopulmonary bypass time, and concerns with the safety of this approach have been raised in the past, however several studies have shown favourable results while utilising this approach in selected cases. 24
This trend of implanting larger surgical valves can already be seen in several hospitals, with one recent report showing that from 2002 to 2012, the average valve label size has increased from 22.8 to 23.9 mm, with the internal diameter increasing from 19.6 to 20.3 mm. 25 While this trend is promising, further research into surgical valve size, type, positioning and techniques allowing for larger valve sizes to be implanted is needed in order to prevent PPM and to improve potential future VIV procedures.
VIV Considerations
Other than the considerations of size and mechanism of failure of the original surgical valve, the type, size and deployment position of the THV are also important in optimising haemodynamic outcomes. Currently, there are two main valve designs: intra-annular valves, where the leaflets are built at the level of the annulus; and supra-annular valves, where the leaflets are above the annulus. Among the commerciallyavailable surgical THVs, the Edwards Sapien XT and Sapien 3 (Edwards Lifesciences) are intra-annular and the Medtronic CoreValve (Medtronic) is supra-annular.
Due to the fact that supra-annular devices have functional areas that operate in a more elevated position, it is expected that they will attain a more complete expansion with better haemodynamic results. This expectation is supported by data from the VIVID Registry, which shows that in small surgical valves with an internal diameter <20 mm, elevated gradients were found in 59 % of Sapien valves, but only 20 % of CoreValves. 9,10 This evidence is supported by an in vitro study that found that the gradient across the Sapien THV is highly dependent on surgical valve size, with a mean gradient of 9.1 mmHg in a 23-mm Carpentier-Edwards Perimount (Edwards Lifesciences, Irvine) surgical valve, 19.5 mmHg in a 21-mm bioprosthesis, and 46.5 mmHg in a 19-mm bioprosthesis. 26 This relationship with surgical valve size is not as significant in CoreValve implants, which is likely due to the supraannular design of the valves consequently allowing for a larger orifice and less restriction from the surgical valve.
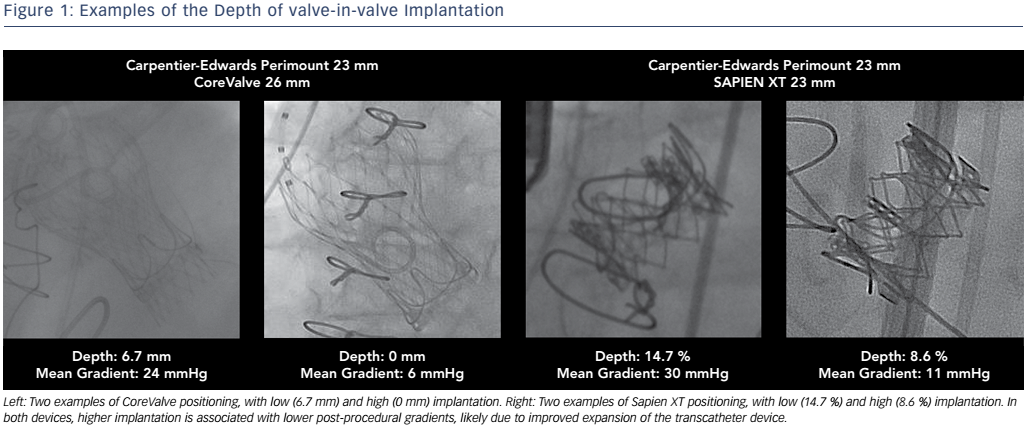
In addition to the type of THV valve, the implantation depth has been shown to be the most important independent factor for elevated mean gradient in a recent VIVID Registry study, followed by the THV valve type and mechanism of surgical valve failure. 27 Other in vitro studies found that the supra-annular position was superior to the annular position in producing lower gradients for the CoreValve in the Trifecta (St. June Medical Inc.), surgical valve. 28,29 Even in a 19-mm surgical valve, it has been shown in vitro that 3- or 6-mm supra-annular deployment of the THV significantly decreases the mean gradient as compared to normal position, but may increase the risk of leaflet thrombosis and valve migration. 30
In a clinical study it was found that high implantation of a THV was associated with lower rates of elevated gradients compared to low implantation, with the optimal implantation depth defined as 0–5 mm for CoreValve Evolut and 0–2mm (0–10 % of device frame) for Sapien XT. 27 While a higher implantation depth would allow for better haemodynamics, it is also important for operators to be aware of the potential risks of an excessively high position, which may lead to coronary obstruction, malpositioning or embolism. 31 The intricacies of optimal THV positioning demonstrate the importance of understanding the different fluoroscopic markings of the surgical valves for determining the ideal position (see Figure 1).
In terms of less conventional approaches, and also considering the importance of using a large THV to improve post-operative haemodynamics, some reports of success through cracking the bioprosthesis ring with ultrahigh-pressure balloons to facilitate the expansion of THV have recently been published. 32,33 While this technique may be valuable in certain contexts, it may be involved with an increased risk of root injury and should be further studied.
The introduction of small THV devices such as the 20-mm Sapien XT/ Sapien 3 and 23-mm CoreValve Evolut may allow for less THV deformation when implanted in smaller surgical valves. 6 The smaller THVs may also create PPM depending on patient characteristics, however, and careful pre-procedural calculations should be conducted. Additionally, an interesting in vitro report recently suggested that THV undersizing may decrease the risk of coronary obstruction in at-risk patients. 34 It is still unclear, however, if the proposed effects are true in real-life situations.
Post-VIV
Care to prevent structural valve degeneration after VIV is another important consideration in improving haemodynamics. Antimineralisation treatment has been reported to be the most significant independent predictor of structural valve degeneration. 35 In addition, a large-scale THV study recently found the absence of anticoagulation therapy at discharge to be an independent predictor of THV haemodynamic deterioration, which illustrates the importance of antithrombotic therapy post-procedure. 36 The standard regimen is warfarin for the first 3 months only, which can be switched to aspirin unless patients have risk factors for thromboembolism such as atrial fibrillation, left ventricular dysfunction and a hypercoagulable condition.
Post-operatively, it is crucial for patients to receive proper assessment and follow-up, including a 30-day echocardogram assessment to allow for the early identification of issues such as elevated gradients and to assess thrombosis risk. Valve thrombosis is a potentially serious complication that is rarely reported and is potentially under-recognised. While most studies of valve thrombosis were analysed for transcatheter aortic valve implantations (TAVIs), there have also been recent reports of thrombosis in VIV procedures. 37 A dramatic increase in mean gradient within 30 days of VIV is a warning for potential thrombotic complications, and these patients should be monitored carefully.
In a systematic review and a large-scale clinical study on valve thrombosis in TAVI, it was found that while the thrombus could not always be visualised with echocardiography, these patients had common features of progressive exertional dyspnoea (65 %), a rapid dramatic increase in mean gradient from 10 to 40 mmHg within months of the procedure, and leaflet thickening (77 %). 38,39 In these two analyses, 81–88 % of the patients were able to restore the mean gradient to baseline within 2 months after treatment with warfarin, while the remaining patients had to receive either percutaneous VIV or surgical valve explantation due to this complication. 34,35 This shows the reversible nature of valve thrombosis if treated promptly; since the median time for valve thrombosis diagnosis is 6 months, it raises the question of whether early detection from proper followup assessments and 30-day echocardiography would be able to prevent additional invasive procedures due to valve thrombosis. 38 Future anticoagulation trials would also help guide future postoperative coagulation protocols and help prevent post-procedural complications such as thrombosis.
Conclusion
The combination of surgical valve characteristics, THV characteristics and implantation depth influence haemodynamic and patient outcomes. Since an elevated gradient is seen in almost a third of VIV patients and remains an unresolved issue, further research into the optimal procedure is needed. Many recent studies have shown the benefits of using supra-annular THVs, which are larger when possible and indicated, and a higher implantation depth in order to reduce the incidence of elevated mean gradients. Other future considerations surround the characteristics and procedure of the initial surgical valve implantation, such as the implantation of larger surgical valves with larger internal diameters to prevent PPM, and incorporation of standardised fluoroscopic markings to facilitate future VIV procedures. With greater understanding of the technical considerations and surgical planning, VIV procedures could be more effective for our increasingly ageing and comorbid population with failed surgical bioprostheses.