The prevalence of heart failure (HF) continues to rise, driven by an ageing population, increasing rates of obesity and diabetes, and better survival in patients with cardiovascular disease.1 While HF is associated with substantial morbidity and mortality, a number of treatments have been shown to improve outcomes in large-scale randomised controlled trials (RCT), including pharmacological inhibition of the renin–angiotensin system (with or without neprilysin inhibition), betablockers, mineralocorticoid receptor antagonists, sinus node inhibition, cardiac resynchronisation therapy with biventricular pacing, implantable cardioverter defibrillators and multidisciplinary models of care.2–4 In order to realise the benefits of these treatments, however, we need to consider how patients were selected for these RCT and how the treatments were delivered.
The adoption of innovative models of care and the delivery of device therapies can be particularly challenging, given that they are highly dependent on the individual operators and rely on substantial infrastructure and staffing support. At first glance, it would appear that the administration of pharmacological therapies for HF should be relatively straightforward, but such therapies are also highly dependent on the individual prescribers, rely on substantial infrastructure and staffing support, and involve real complexities around medication persistence and patient adherence.
In this review, we will discuss medication titration in HF and consider whether or not it is too complex in the real-world setting. We conducted a literature search in November and December 2016 using PubMed. Keywords included ‘heart failure’, which was used in combination with ‘medication’ or ‘therapy’ and ‘titration’ or ‘up-titration’, ‘dose’ or ‘target dose’. We also searched reference lists of relevant primary studies and systematic reviews. We used no language or date restrictions. All types of study design were included where medication titration was a primary endpoint.
Clinical Trials Evaluating Medications in Heart Failure
Large-scale randomised controlled trials (RCTs) have demonstrated that angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), beta-blockers and mineralocorticoid receptor antagonists all improve clinical outcomes in patients with HF associated with a reduced left ventricular ejection fraction (HFrEF).5–12 Indeed, such studies suggest that the combination of an ACEI, betablocker and mineralocorticoid receptor antagonist should translate to a 60–70 % relative risk reduction in all-cause mortality.13 Nevertheless, it is likely that the balance between benefit and harm varies according to patient age, disease severity, associated comorbidities and approaches to monitoring and medication titration. An example of the impact of comorbidities was demonstrated by an individual patient data meta-analysis of the beta-blocker RCTs, in which the benefits were only observed in patients in sinus rhythm, there was no reduction in hospitalisation or mortality in patients who were in atrial fibrillation when they were enrolled in the studies.14
Clinical Trial Evidence for Target Doses
In the absence of an easily measurable and accepted HF physiological surrogate endpoint, the large HF RCTs evaluating the efficacy of ACEIs, ARBs and beta-blockers used forced up-titration at pre-specified intervals, unless there were adverse events or intolerance, aiming for target doses largely guided by those used to treat hypertension. Early studies reported conflicting results as to whether ACEIs have a dose–response effect on haemodynamic measures in HF patients.15,16 The NETWORK investigators compared three doses of enalapril in 1,532 HF patients and failed to demonstrate a dose-related difference in the combined endpoint of death, HF-related hospitalisation or worsening HF.17 A reduced left ventricular ejection fraction was not a prerequisite for patient selection, however, and patients were only followed for 24 weeks. The Assessment of Treatment with Lisinopril and Survival (ATLAS) study is the largest to address the question of whether we should aim for higher doses of ACEIs. In this study, 3,164 HFrEF patients were randomised to receive either low-dose (2.5–5.0 mg/day) or very-high-dose (32.5–35.0 mg/day) lisinopril.18 While there was no significant difference in the primary endpoint of all-cause mortality, there was a significant 12 % relative risk reduction in the combined endpoint of all-cause mortality or hospitalisation, which was driven by a 24 % relative risk reduction in HF hospitalisation. This study was originally powered based on the assumption that low-dose lisinopril would have no effect on mortality. Indirect comparisons, however, suggest that the low dose achieved approximately half the reduction in mortality and HF hospitalisation observed with the doses of enalapril used in the Studies of Left Ventricular Dysfunction (SOLVD)–Treatment research.10 The authors concluded that patients should be titrated beyond low doses of ACEIs unless the dose is limited by side effects.18 While it was unclear whether patients should be titrated to the very high dose of ACEI used in the ATLAS study, the difference between intermediate and high doses seemed to be small.
Indirect comparison of the initial Cardiac Insufficiency Bisoprolol Study (CIBIS) and CIBIS-II provides some support for the use of higher doses of beta-blockers.5,19 CIBIS (up to 5 mg/day bisoprolol without forced up-titration) failed to achieve its primary endpoint, with a non-significant 20 % relative risk reduction in mortality; however, CIBIS-II (forced up-titration to 10 mg/day bisoprolol) demonstrated an impressive 34 % relative risk reduction in mortality in HFrEF patients. Subsequent post-hoc analyses from the Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart failure (MERIT-HF) study and CIBISII reported similar placebo-corrected benefits regardless of the dose of beta-blocker achieved.20,21 These were non-randomised comparisons, however, and are therefore subject to selection bias. Furthermore, based upon the trial design, all patients had been exposed to forced up-titration to the pre-specified target dose and were therefore on maximal tolerated doses. The Multicenter Oral Carvedilol Heart Failure Assessment (MOCHA) study reported dose-related, placebo-corrected improvements in left ventricular ejection fraction and survival in patients who were randomised to receive 25 mg twice daily carvedilol compared with lower doses; however, this analysis was based upon 25 deaths.22 While this was a small study, it nonetheless provided further support for maximal tolerated doses of beta-blockers.
In summary, the majority of studies or analyses that have been undertaken to address the question of whether lower doses of ACEIs and beta-blockers achieve similar benefits were either underpowered for major clinical outcomes or based on post-hoc, nonrandomised comparisons. Nonetheless, the major efficacy studies were all based upon forced up-titration aiming for specified target doses. For this reason, clinical guidelines recommend up titrating to maximal tolerated doses.2–4
Target Dose Achievement in Clinical Practice
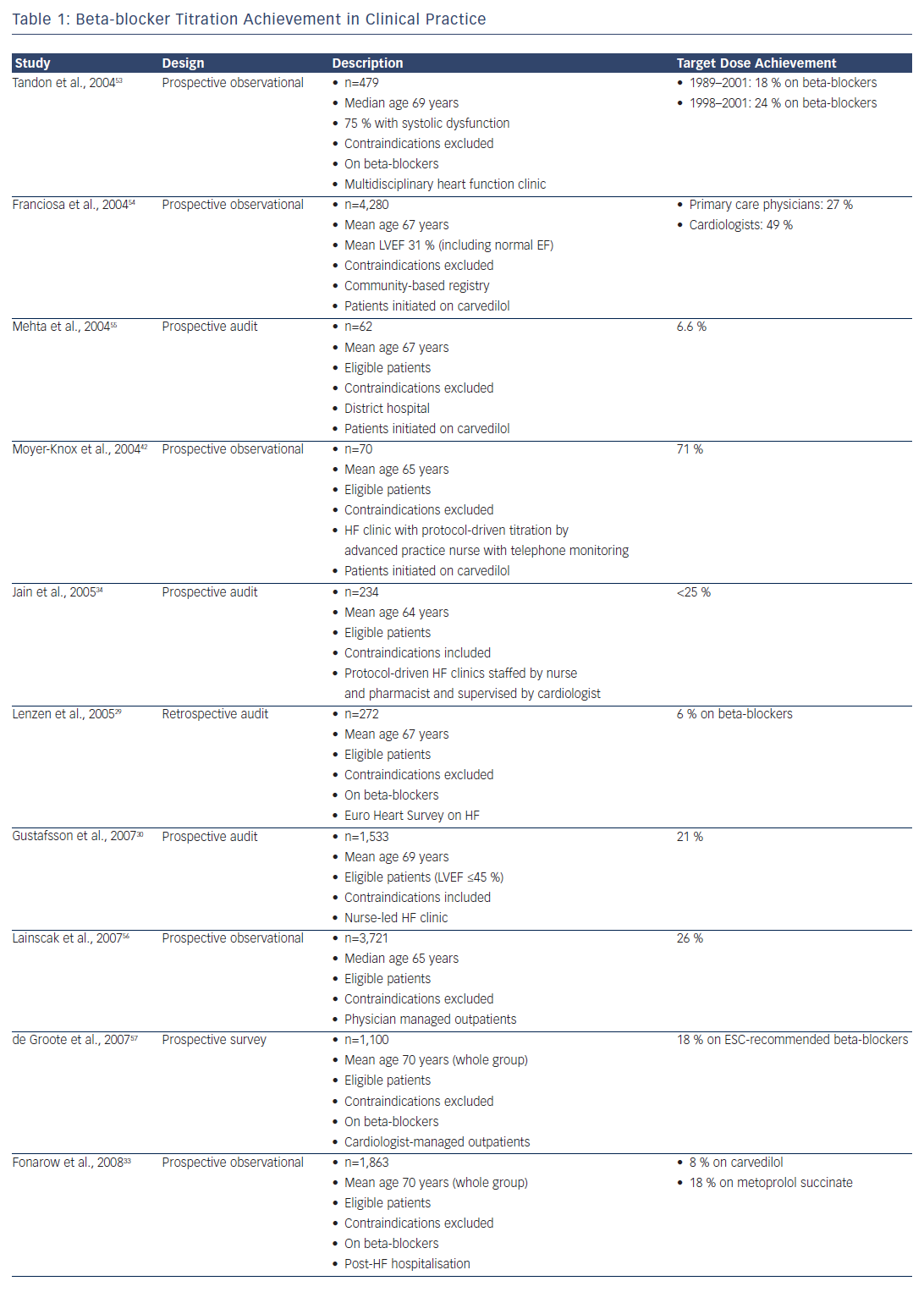
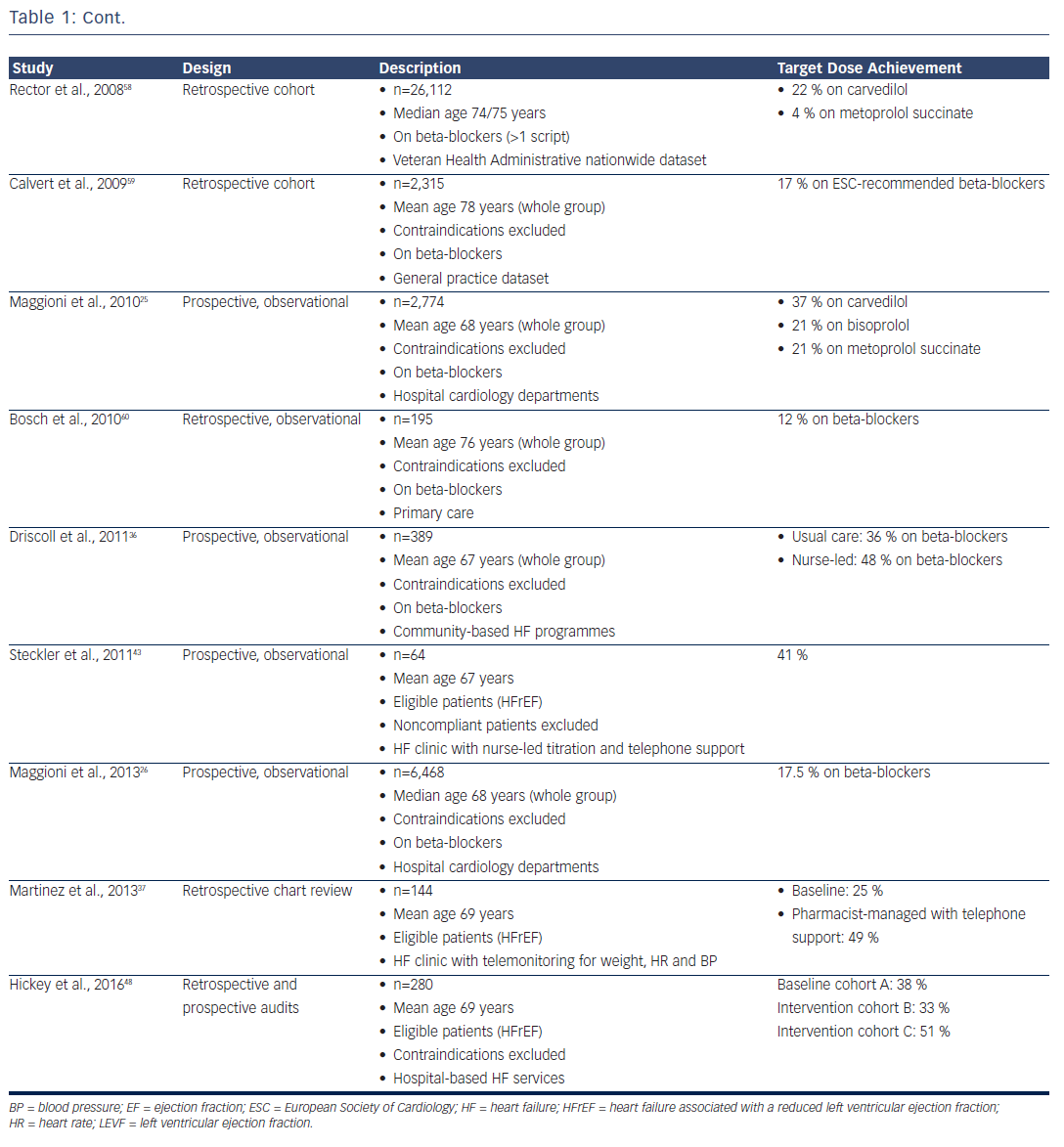
HF prescription rates for both ACEIs/ARBs and beta-blockers have increased substantially over the past two decades,23–26 such that 92 % of patients were on ACEI or ARB therapy and 93 % were on beta-blockers in the recent European Society of Cardiology Heart Failure Longterm Registry, with most of those patients not on treatment having a documented contraindication or previous medication intolerance.26 Despite this, only 29 % of patients were on target doses of ACEIs and 18 % were on target doses of beta-blockers, with approximately onethird having no reason documented for the failure to up-titrate.26 This contrasts with the RCTs, where at least 50–60 % of patients achieved target doses.5,6,10–12
Clinicians have generally paid greater attention to the up-titration of beta-blockers, given the impressive benefits achieved in clinical trials that involved the forced up-titration of these drugs to target doses on top of background therapy (which included an ACEI or ARB in >90 % of patients).5,6,11,14 Furthermore, while ACEIs can be safely up-titrated in a relatively short time period,27 a ‘start low and go slow’ approach is generally taken with beta-blockers, given their short-term, negative inotropic effects. Despite the benefits, however, only 10–30 % of patients achieve target doses of beta-blockers in most real-world studies (see Table 1).
One reason for the low titration rates achieved in clinical practice may be that the patients are not selected in the same way as those enrolled in clinical trials; in practice, patients are generally over a decade older with numerous comorbidities. Indeed, after applying all the RCT inclusion and exclusion criteria to the Euro Heart Survey on Heart Failure population,28 only 9 % of patients would have been eligible for enrolment in the SOLVD–Treatment study and 5 % in the MERIT-HF study.29 Even in the patients who satisfied all the eligibility criteria for those studies, only 46 % achieved target doses of ACEIs and just 6 % achieved target doses of beta-blockers.29 This outcome suggests that the systems available to support clinical trials (including the availability of dedicated research nurses applying forced medication up-titration under the supervision of a principal investigator) may not be readily available in routine clinical practice.
Medication Titration Intervention Studies
Barriers to medication titration include health-provider knowledge, self-efficacy and attitudes; patient-related factors, including age, body mass index, comorbidities and polypharmacy; and limited time and support structures to facilitate regular monitoring.30–32 Patients also frequently transition between the acute and community healthcare sectors, which further complicates care coordination, as there is unclear role delineation for healthcare providers. This was clearly demonstrated in the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure, where there was no attempt to up-titrate beta-blocker therapy in over two-thirds of patients in the initial 60–90 days following hospital discharge.33
A number of strategies have been evaluated to improve medication prescribing in HF, including case management, educational initiatives, decision support, telephone-based monitoring, clinical audit and feedback, strategies to improve communication between healthcare providers and extended scope of clinical practice.30,34–44 While education, decision support and clinical audit and feedback have been successfully applied to improve prescribing behaviour, these approaches alone appear to be insufficient to improve medication titration.39,40 Successful strategies have generally involved multifaceted interventions and are likely to be context-specific (see Table 2).
On the basis of clinical studies demonstrating the efficacy of multidisciplinary HF disease management, these programmes are becoming more widely available.45,46 Indeed, those strategies used in the larger HF RCTs that have demonstrated the efficacy of pharmacological therapies are replicated in many HF disease-management services. These include the availability of dedicated nursing staff that undertake regular follow-up and monitoring of their patients and also provide a convenient point of contact. Such staff members could also be engaged to undertake ‘forced medication up-titration’. Recent studies have therefore evaluated the role of expanding the scope of nurses’ and pharmacists’ clinical practice for medication prescribing and titration, with favourable results reported in a number of prospective, observational studies.30,34,36,42,43
Ansari et al. undertook a single-centre, three-arm RCT in the United States comparing nurse-facilitated medication titration, provider and patient notification, and standard care in 169 HFrEF patients.39 All three groups received copies of HF treatment guidelines and group education. The nurse-facilitated medication titration group was much more likely to achieve target doses of beta-blockers at 12 months (43 %), compared with both the provider/patient notification group (2 %) and standard care (10 %). These findings are largely consistent with an evaluation of Australian community-based HF services that reported a higher proportion of patients achieving target doses of beta-blockers if they were enrolled in programmes undertaking nurseled medication titration.36 Similar improvement was reported in a RCT involving a multifaceted intervention including nurse-coordinated case management, education and telephone-based structured monitoring.44 A recent Cochrane review encompassing seven RCTs performed in 1,684 HFrEF patients reported that nurse-led medication titration resulted in a two-fold increase in the number of participants achieving target doses of beta-blockers, a 20 % relative risk reduction in all-cause hospitalisation and a 34 % relative risk reduction in all-cause mortality. These findings need to be tempered, however, by the lack of reporting of harm, with only two studies reporting on adverse events.47
Studies have also evaluated pharmacist-managed medication titration in HF.37,38 Recognising the key role that general practitioners (GPs) play in HF management, Lowrie et al. undertook a cluster-RCT of nonspecialist, pharmacist-managed medication titration in 1,090 HFrEF patients attending 87 practices in United Kingdom. While this study did not achieve its primary clinical endpoint, a higher proportion of patients who enrolled in practices with pharmacist-assisted titration achieved target doses of ACEI therapy. There were favourable trends for beta-blocker titration, however these did not achieve statistical significance.38 In a before–after, retrospective design study targeting 51 higher-risk HFrEF patients conducted in the United States, Martinez et al. demonstrated that a multifaceted intervention, which included pharmacist-managed titration guided by telephone monitoring, resulted in a higher proportion of patients achieving target doses of ACEI/ARB and beta-blockers.37
We recently undertook a quality improvement study that aimed to embed a pre-designed medication titration plan into standard clinical practice for patients newly referred to the HF services in three hospitals.48 This study involved completing and faxing a customised medication titration form to the patient’s GP on the day of hospital discharge that clearly defined which healthcare provider was primarily responsible for medication titration. This intervention was designed to facilitate point-of-care decision support, including specifying the order and extent of medication titration and providing trouble-shooting guidelines (see Appendix I). We discovered that patients who were not on target doses of ACEI/ARB or beta-blocker therapy were more likely to achieve target doses if the medication titration form was used. In the final cohort, 55 % of patients achieved target doses of ACEIs/ ARBs and 51 % achieved target doses of beta-blockers 6 months after hospital discharge.48 This medication titration form is now available online (http://www.health.qld.gov.au/heart_failure) and is used by all public hospitals with HF services in Queensland, with similar ACEI/ARB and beta-blocker titration rates observed in a recent analysis of an independent HFrEF cohort.32
A major limitation of strategies that are either coupled with established HF disease management programmes or that require additional staffing is their sustainability and limited external validity, since access to multidisciplinary HF services varies across jurisdictions.49 Given the central role played by the GP, processes that engage primary care are more likely to be successful in the broader HF population. Some studies, however, have reported a reluctance on the part of GPs to up-titrate HF therapy.50 Despite this, we observed increasing primary care involvement in our quality improvement study, with the patient’s GP being the designated healthcare provider responsible for medication titration in half the patients in the final cohort, when the highest titration rates were achieved.48 This suggests that, in addition to long-term monitoring and care coordination,51,52 GPs could play a more active role in medication titration.
Conclusion
There have been marked improvements in ACEI and beta-blocker prescription rates for HFrEF over the past two decades; however, the doses prescribed in clinical practice are generally much lower than those achieved in the RCTs. While the clinical trials were generally not designed to determine whether the benefits were dose related, the successful studies were nonetheless based on forced up-titration to pre-specified target doses. Clinical audit and feedback have been shown to improve prescribing behaviour; however, additional contextspecific measures are required to support medication titration. The scope of clinical practice for nurses and pharmacists has successfully been extended to medication titration and builds on the recognised benefits of multidisciplinary HF disease management. However, strategies that engage primary care with timely communication, clear role delineation and point-of-care decision support may have wider applicability to allow the impressive gains demonstrated in the clinical trials to be applied to the broader HF population.