The left atrial appendage (LAA) is a trabeculated structure with variable anatomy comprised of pectinate muscle, which grows out of the primary atrium before the left atrium (LA) develops. This outcropping joins the venous component of the LA by way of a bottlenecked junction, in contrast to the anatomy of the right atrial appendage which, although trabeculated, joins the atrium via a wider neck.1
Although the exact function of the LAA is unknown, morphologic studies suggest it may play a role in offloading atrial pressure during left ventricular systole.2 Left ventricular rate is inversely correlated with LAA emptying during early diastole, which is hypothesized to play a role in the development of thrombus in the LAA in patients with non-valvular AF (NVAF).3
Transesophageal echocardiography (TEE) is the standard method of evaluating the size and shape of the LAA and to exclude thrombus within it. The Assessment of Cardioversion Using Transesophageal Echocardiography (ACUTE) trial demonstrated that using TEE results in decreased time to cardioversion and the incidence of both major and minor hemorrhagic events in patients with NVAF found to have no LAA thrombus. The reduction in hemorrhagic events was attributed to a lack pre-cardioversion anticoagulation in the no-thrombus population.4 Significantly, even if pre-cardioversion TEE shows no thrombus, post-cardioversion anticoagulation remains essential to prevent embolic complications, possibly due to fibrillatory-induced atrial and appendageal myopathies.5,6
TEE findings of increased LAA size and irregular orifice flow patterns are associated with an increased risk of LAA thrombus.7,8 Thrombus characteristics such as size, mobility and shape can also be assessed on TEE and correlated with embolic risk.9
The LAA is the site of thrombus formation in more than 90 % of patients with NVAF, making it an important therapeutic target to reduce embolic stroke risk.10 Unfortunately, pharmacological and catheter-based interventional strategies to abort AF have not been shown to reduce stroke risk.11 Meta-analysis supports the use of anticoagulation therapy in patients with NVAF, especially in subgroups determined to be at high risk based on the CHA2DS2–VASc score.12
Given the potential complications of long-term anticoagulation medication, there are patients in whom the risks of anticoagulation therapy outweigh the potential benefits of ischemic stroke prevention, such as those with prior intracerebral hemorrhage. As a result, non-pharmacological strategies aimed at reducing embolic risk in patients with AF have been developed.
Closure Strategies: Percutaneous, Thoracoscopic, and Open
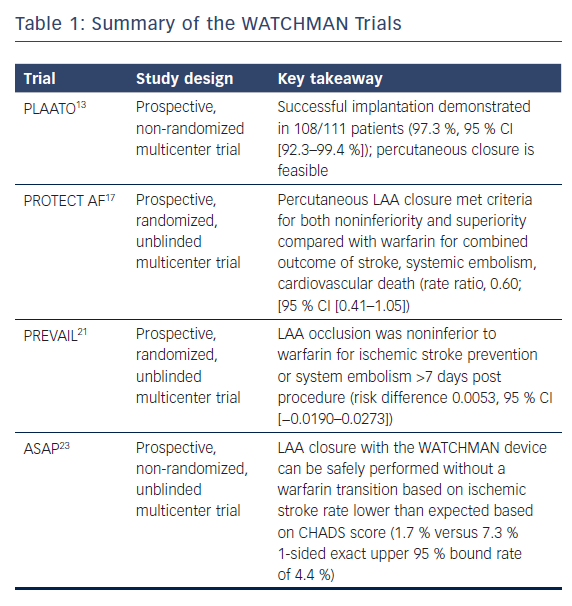
The Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO) study in 2002 was the first to demonstrate that a device could be used to close the LAA.13 Five-year follow-up data showed that percutaneous devices were effective in reducing stroke risk in patients with NVAF by 2.8 % per year compared to the cumulative risk predicted by CHADS2 score, and provided the impetus for further development of percutaneous closure devices.14
The most widely used endovascular percutaneous devices are the WATCHMAN (Boston Scientific) and Amplatzer Amulet (St Jude Medical). Only the WATCHMAN has obtained FDA approval for implantation in the US, although multiple devices are in varying stages of development. The placement of these devices requires femoral venous access with transseptal puncture to access the LAA for implantation.
Other closure devices may be placed via the pericardium, some of which require surgical thoracotomy incision to deploy the device. The LARIAT (SentreHEART), AtriClip (AtriCure) and Tiger Paw II (Maquet Cardiovascular) all have FDA approval for transpericardial LAA closure, with the latter two requiring surgical thoracotomy.
Surgical ligation or amputation of the LAA when performing other cardiac surgery is an invasive method of LAA exclusion. The efficacy of surgical LAA exclusion/removal has been called into question, however. A 2008 retrospective analysis of 137 patients undergoing surgical exclusion or excision of the LAA found that follow-up TEE (approximately 8–20 months postoperatively) demonstrated a failure rate of up to 60 % assessed by Doppler flow within the appendage or remnant. Notably, there was no significant difference in stroke risk between the successful and the non-successful surgical closure groups.15 A more recent study from the Mayo Clinic found in a large, propensity-matched analysis that prophylactic surgical LAA exclusion during cardiac surgery had no effect on long-term risk of stroke or mortality during follow-up.16
Percutaneous Left Atrial Appendage Occlusion Devices
WATCHMAN
The Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) trial was a non-inferiority trial comparing warfarin therapy to WATCHMAN implantation in patients with permanent or paroxysmal NVAF who had no contraindications to oral anticoagulation. The trial randomized patients to either oral warfarin or WATCHMAN implantation, followed by a 45-day course of warfarin, and then clopidogrel and aspirin for 6 months, followed by indefinite aspirin monotherapy. A composite primary efficacy endpoint of stroke, cardiovascular death, and systemic embolism was measured in addition to the primary composite safety endpoint of major bleeding, pericardial effusion, and device embolization.
At the 1,065 patient-year follow-up, WATCHMAN was found to be non-inferior, as measured by the primary efficacy endpoint (3.0/100 patient-years in the intervention group versus 4.9/100 patient-years in the control group). Notably, the primary safety endpoint was significantly more frequent in the intervention group (7.4/100 patient years in the intervention group versus 4.4/100 patient years in the control group).17 Four-year follow-up data confirmed the primary endpoint non-inferiority and superiority and suggested a significantly lower rate of cardiovascular and all-cause mortality in the intervention group.18
A subsequent analysis compared the PROTECT AF cohort to the open label Continued Access Protocol registry and noted significant decline in the primary safety endpoint within 7 days of the implantation procedure (7.7 % and 3.7 %, respectively).19 The same analysis showed that the intra-trial primary safety endpoint declined between the first and second half of the trials, implying that increased operator experience improved the safety of WATCHMAN implantation. This finding was again suggested in a comparison to the EWOLUTION registry, in which device deployment was successful in 98.5 % of high-risk patients and 30–day periprocedural mortality was 0.7 %.20
The Prospective Randomized Evaluation of the WATCHMAN LAA Closure Device In Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy (PREVAIL) trial was another randomized evaluation of the WATCHMAN device, which used the same post-implantation anticoagulation regimen in a similar population to the PROTECT AF trial. The trial found the WATCHMAN device was non-inferior to warfarin in preventing stroke or systemic embolism within 7 days of implantation but did not meet statistical significance with regards to the combined primary efficacy noninferiority endpoint of stroke, systemic embolism and cardiovascular/unexplained death. The trial also found safety was better than in the PROTECT AF trial.21 Based on these findings, the WATCHMAN was granted FDA approval in March 2015. A recent meta-analysis of the PREVAIL and PROTECT AF trials with 5-year follow-up found statistically significant reductions in hemorrhagic stroke, post-procedure bleeding and both cardiovascular and all-cause mortality in the groups that underwent LAA occlusion.22
The ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology (ASAP) study was designed to assess the effectiveness of the WATCHMAN implant in patients with stronger contraindications to anticoagulation with warfarin (noted as hemorrhagic/bleeding tendencies, blood dyscrasias, senility, high fall risk and other documented reasons such as hypersensitivity). Device implantation was followed by 6 months of a thienopyridine antiplatelet agent and lifelong aspirin. The study found a reduction in stroke rate to 1.7 % from the 7.3 % predicted by the mean CHADS2 score of patients in the ASAP registry (2.8 ± 1.2), suggesting that implantation can be safe without warfarin transition.23
One substudy of the PROTECT AF study reported a 32 % incidence of flow seen around the device at 12 months.24 The presence of peridevice flow at 1 year was not associated with increased risk of clinical thromboembolic events. Stroke patients with peridevice flow leak were more likely to have continued on anticoagulation therapy. Device-associated thrombus (DAT) was a rare complication and was related to AF burden, size of implanted device and adherence to the postprocedural anticoagulant regimen. Warfarin therapy after the discovery of device-related thrombus achieved complete resolution in retrospective analysis.25 Table 1 summarizes several of the large WATCHMAN trials.
Amulet
The Amulet device is an alternative to the WATCHMAN and has gained CE marking but no FDA approval. It has a different occluder structure from the WATCHMAN. The Amulet has been found to significantly reduce the annual rate of systemic thromboembolism in patients with NVAF compared to the rate predicted by their CHA2DS2–VASc score.26
Device-associated Thrombus
One recent pooled meta-analysis suggests that DAT prevalence affects all devices at a rate of 3.9 % and can be broken down by device – 3.4 % for WATCHMAN (40/1,184), 4.6 % for ACP/Amulet (35/757), 4.8 % for ACP (34/707).27 Recent multicenter data from 1–2 years follow-up suggest that DAT has a strong association with risk of ischemic stroke (HR 4.39, 95 % CI [1.05–18.43]), and that protective factors include dual antiplatelet therapy (HR 0.10, 95 % CI [0.01–0.76]) and oral anticoagulation (HR 0.26 95 % CI [0.09–0.77]).28
Further randomized controlled trials are necessary to determine the optimal post-implantation anticoagulation regimen for better characterization of device-associated risk as to best avoid iatrogenic complication of device implantation.29
Percutaneous Epicardial Left Atrial Appendage Exclusion Devices
LARIAT
The LARIAT device requires epicardial access and an endovascularly placed magnetic guidewire to ultimately provide suture ligation of the most anterior lobe of the LAA. This device has FDA approval as a tissue closure device, and has been used as a LAA exclusion device. However, retrospective multicenter data have shown a high periprocedural major complication rate (9.7 %) and a significant rate of pericardial effusion (10.4 %) in patients undergoing the LARIAT ligation procedure.30,31 At present, this device is the only nonsurgical option for patients who have an absolute contraindication to oral anticoagulation even for a short duration post-implant. The LARIAT is associated with significant reduction in annual stroke rate compared to what would be expected for the studied population (1 % observed event rate versus 6.2 % expected in one multicenter trial).32
Addition of LARIAT ligation to conventional catheter ablation has been shown in one observational study to reduce persistent NVAF, assessed by the primary endpoint of freedom from antiarrhythmic medication after a single ablation procedure (65 % in the LARIAT ligation group versus 27 39 % undergoing ablation only; p=0.002), possibly due to reduced substrate for propagation of reentry.33
Standalone Thoracoscopic Approaches
Novel thoracoscopic approaches have been developed to achieve minimally invasive surgical LAA exclusion. Ohtsuka et al.34 carried out a standalone thoracoscopic LAA appendectomy in 30 patients with contraindications to standard anticoagulation therapy. The study used 3D CT to assess successful closure of the LAA and there were no thromboembolic events in up to 38 months of follow-up.
A similar procedure for exclusion of the LAA with the AtriClip device demonstrated effective exclusion in up to 93.9 % in one single-center trial. The authors of this study acknowledge that the significance of a remnant stump seen in this procedure remains unknown and TEE evidence of complete occlusion should be obtained before cessation of oral anticoagulant therapy.35
The data on surgical closure of the LAA to prevent thromboembolism are mostly observational and non-randomized. The on-going Left Atrial Appendage Occlusion Study (LAAOS) III will end its enrollment in 2018 and should have a sufficient number of patients to detect any significant reduction in primary outcomes using open surgical closure.36
Conclusion
The LAA remains an important target for thromboembolic prophylaxis in patients with NVAF. There are few multicenter randomized controlled trial data to endorse one strategy over another as the number of options to achieve LAA exclusion continues to grow.
Given the minimal evidence available at present, consensus guidelines make only weak recommendations in support of LAA occlusion.37 The 2012 focused update to the European Society of Cardiology Guidelines for the Management of Atrial Fibrillation gave a class IIb level B recommendation for the use of LAA occlusion devices in patients who have contraindications to long-term oral anticoagulant therapy.38 However, these guidelines were called into question in a 2015 update, which noted that such recommendations were built on studies where patients did not have oral anticoagulant therapy contraindications.39
It is worth noting that both the PROTECT AF and PREVAIL trials were designed as non-inferiority trials in comparison to standard medical anticoagulant therapies. By achieving non-inferiority, these devices show a comparable effectiveness in preventing the known complications of NVAF; however, the decision to use such a device must remain at the level of the patient and provider taking into account bleeding risk and compliance.
Since this update, the WATCHMAN has gained FDA approval, but there has been no consensus statement in the US with recommendations for LAA occlusion with the closure devices described above. Instead, there is a growing number of guidelines calling for more trials and codification of valuable endpoints in keeping with this new, growing technology.40
Another significant consideration is the expanded use of non-vitamin K antagonist anticoagulant therapy, which may be appropriate for patients with contraindications to warfarin anticoagulation. This was suggested in the 2011 Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial.41
There may be a role for intervention if it can be shown in large trials that implant devices benefit patients with risks for anticoagulation. It remains to be shown whether combined therapy with non-vitamin K antagonists and LAA occlusion can provide superior results to either method independently.
As strategies for occlusion improve and periprocedural risks decline, outcome measures must be assessed at the level of the individual patient. Stroke risk ought to be reduced in a way that promotes good compliance and has meaningful quality-of-life benefits to patients with NVAF.