The indications for percutaneous coronary intervention (PCI) have expanded steadily during the past years. After the days of the revascularisation of obstructive coronary artery disease (CAD) by balloon angioplasty,1 the introduction of coronary stents has essentially contributed to PCI being one of the most frequently performed invasive therapeutic procedures worldwide. Bare metal stents (BMS) provided a solution to acute vessel occlusion by sealing dissection flaps and at the same time reducing the risk of restenosis through prevention of acute recoil and inward remodelling.2 The development of drug-eluting stents (DES) solved the problem of in-stent restenosis by reducing neointimal hyperplasia3,4 and recently, bioresorbable stents (BRS) have been developed. These BRS allow for temporary scaffolding of the vessel. Since they are gradually resorbed, they might allow the return of vasomotion, late luminal enlargement and late expansive remodelling. In contrast, conventional metallic stents cage the coronary artery by implanting a permanent foreign body that can lead to non-conformability of the stented vessel.5,6 Acute changes in the geometry of coronary arteries following implantation of these stents have been related to adverse clinical outcomes.7 Furthermore, acute or late strut malapposition as well as the jailing of side branches caused by the metallic cage might be associated with adverse clinical outcomes.8 Malapposition could contribute to thrombosis and a side branch jailed by the metallic cage can jeopardise access to the side branch and potentially promote restenosis. The unique potential advantages of BRS to allow for temporary scaffolding could reduce the risk of adverse clinical outcomes caused by acute vessel geometry changes, late malapposition, jailed side branches or inflexibility of the permanent stent, because of the disappearance of the stent over time.9,10 These unique potential benefits of BRS over conventional metallic stents eventually led to the development of several types of BRS. To evaluate this development and the behaviour of BRS over time, the use of catheter-based intracoronary imaging techniques is gaining momentum. The aim of this review article is to describe the results of using clinically available invasive imaging techniques for the evaluation of BRS and put their contribution into perspective.
Challenges For the Evaluation of Bioresorbable Stents
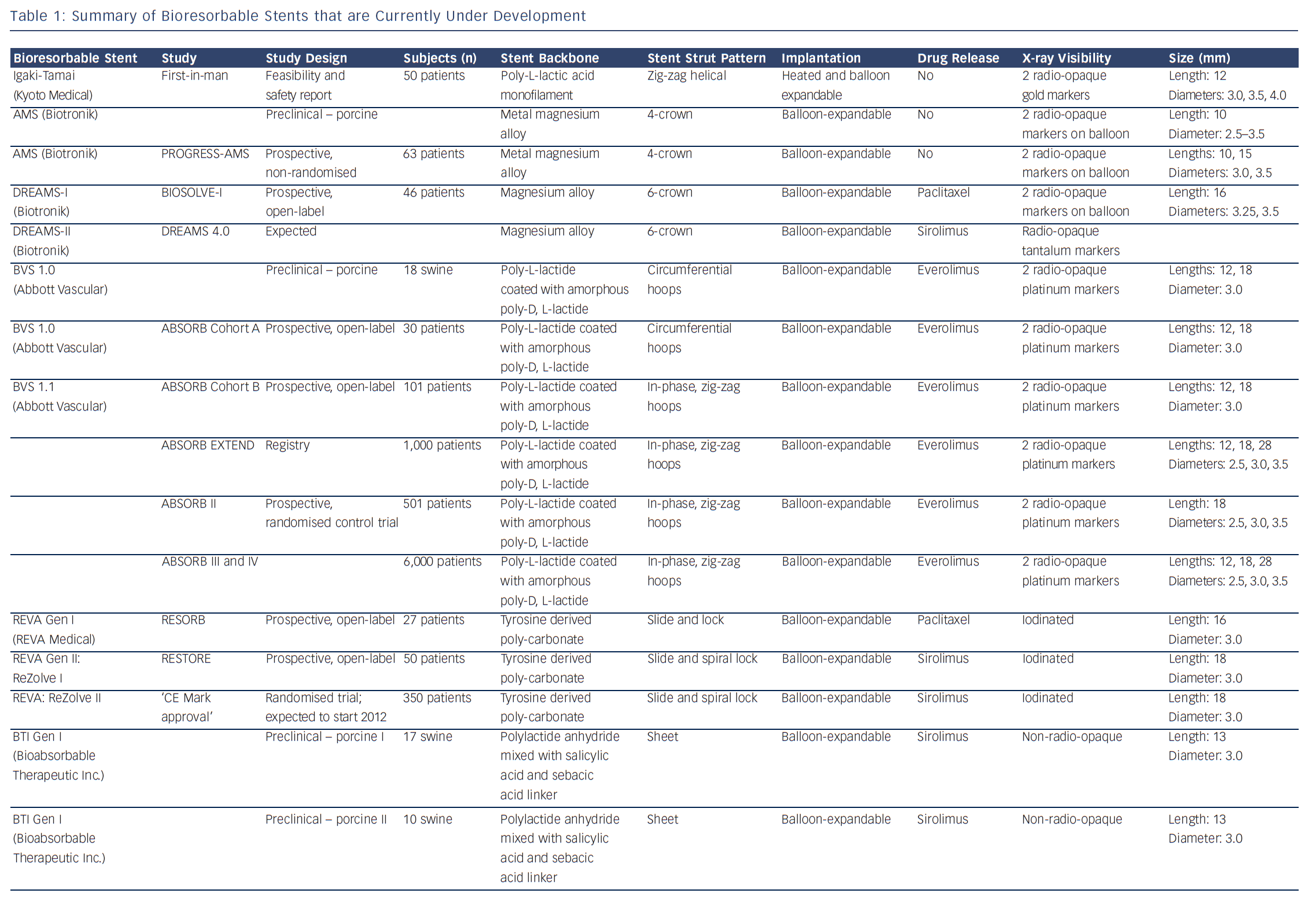
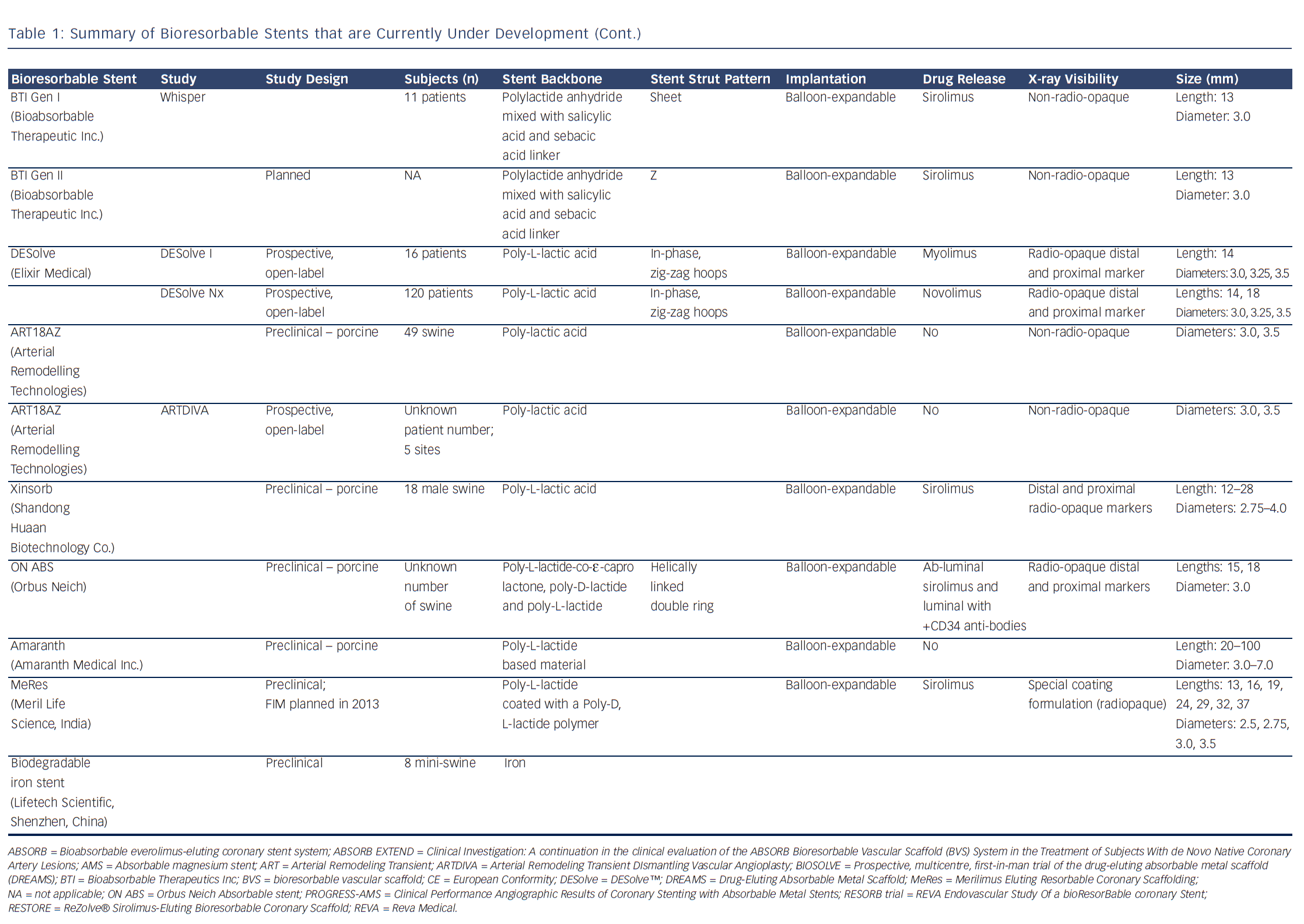
Polymers tend to have substantial different material characteristics compared with metallic stents and thus pose a variety of challenges for application as scaffolds in coronary arteries. Table 1 shows a detailed description of the BRS of which published data is currently available. The majority of BRS have a balloon-expandable design that requires careful sizing to ensure the stent does not crack upon deployment due to over-expansion. Self-expanding scaffold designs11 as well as a scaffold with a slide and lock design12 have also been developed. Most of the BRS are composed of translucent material, and therefore different approaches are used to allow for visualisation under X-ray – stents are equipped with radio-opaque markers on either both ends of the stent or on both ends of the balloon, or a proprietary iodinated material is added to the polymer, allowing visualisation of the complete stent. As a result of the non-radio-opaque design of most of these devices, angiography has limited sensitivity to diagnose stent under-expansion or evaluate stent degradation. Limitations of coronary angiography as a luminogram technique, the limited spatial resolution and the inability to visualise non-radio-opaque structures could be overcome using high-resolution catheter-based intravascular imaging techniques such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT).13,14 Furthermore, to evaluate the chemical composition of the tissue behind or on top of the stent, near-infrared spectroscopy (NIRS) could be used. These high-resolution diagnostic modalities allow for detailed evaluation of the vessel over time. Before BRS-implantation, intracoronary imaging can help determine the appropriate stent size in terms of diameter and length because of the ability to visualise the extent of coronary plaque and side branches. Vascular injury caused by the implantation procedure can be accurately evaluated and furthermore, the expansion, apposition, mechanical integrity and resorption process of the stent as well as the position with regard to side branches can be visualised. Moreover, of the translucent BRS, the underlying plaque can be evaluated.
Intracoronary Imaging of Bioresorbable Stents Using Clinically Available Techniques
Greyscale Intravascular Ultrasound
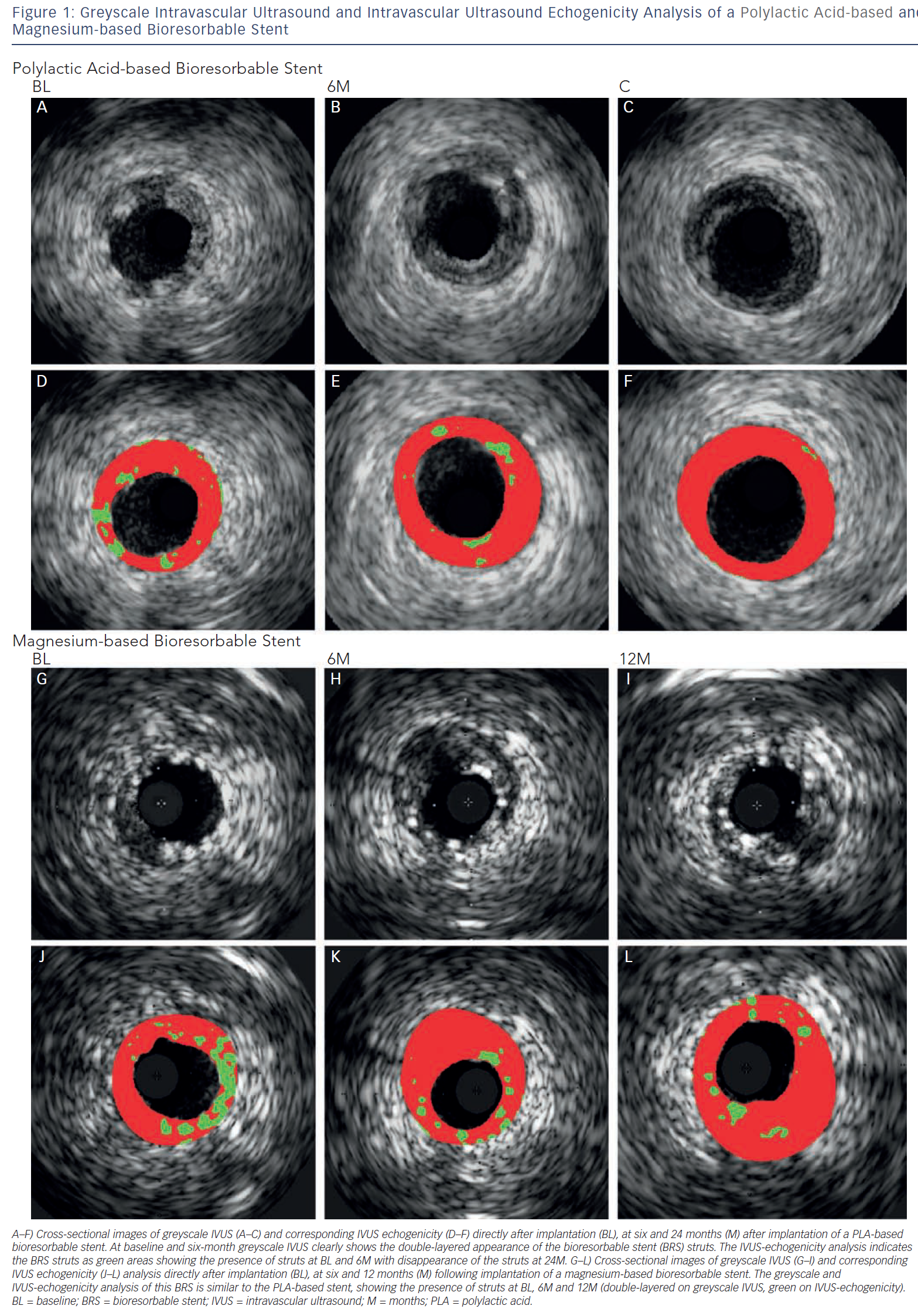
IVUS is an intravascular imaging technique that provides realtime high-resolution images of the vessel wall and treatment devices. Depending on the distance from the catheter, the axial resolution is approximately 80–150 micrometers, the lateral resolution 200–300 micrometers. Based on the echogenicity and the thickness of the material, different features of the vessel wall, treatment devices and the coverage of these devices can be evaluated.15 IVUS can adequately identify the lumen and vessel contours, allowing for the evaluation of plaque burden and vessel remodelling. Calcified components can be detected by IVUS with high accuracy; however, the ultrasound is backscattered by calcium causing acoustic shadowing, and therefore it is impossible to evaluate the extent of the calcium or evaluate underlying structures. The polymeric struts of BRS have an echogenic intensity similar to calcified components without acoustic shadowing, allowing for visualisation of the struts. They are depicted as hyper-refractive boxes with an echogenic blooming effect, causing a double-strut appearance on IVUS (see Figure 1). This allows for the assessment of the short- and long-term outcome of BRS and the treatment failures, such as strut malapposition, restenosis or stent thrombosis. However, the resolution of IVUS limits the assessment of the dimensions of thrombi and therefore makes guidance of their removal difficult. Furthermore, greyscale IVUS has a limited ability to make a distinction between morphological components of plaque, e.g. between lipid-rich and fibro-lipid plaque, and to quantify the degradation of BRS struts due to visual assessment. To assess morphological components of plaque and degradation of the BRS, IVUS-based imaging techniques such as IVUS-virtual histology (IVUS-VH), which classifies plaque into four components, and IVUS-echogenicity, which calculates the relative fraction of hypoechogenic versus hyperechogenic tissue volumes, could be used. Furthermore, for the treatment of thrombosis, OCT with its higher resolution, could be a very attractive imaging modality.
Intravascular Ultrasound Virtual Histology
IVUS-VH is the first available IVUS backscattering image analysis system built on the 20 megahertz (MHz) phased array IVUS platform. Data acquisition is similar to standard IVUS but data analysis is performed on dedicated consoles. IVUS-VH is mainly used for tissue characterisation and classifies plaque into four components that are labelled with a specific colour – calcium appears white, fibrous tissue green, fibrolipidic tissue greenish-yellow and necrotic core red.16,17 The ability to visualise the full circumference of the lumen and vessel wall is similar to conventional greyscale IVUS. However, conventional IVUS has limited ability to discriminate between plaque composition and to assess BRS strut degradation. IVUS-VH depicts most stent struts as dense calcium artefacts and thus allows for estimation of the stent degradation by comparing the amount of dense calcium directly after implantation with follow-up. Since the polymeric struts do not cause acoustic shadowing, the composition of the plaque behind the stent can be evaluated.18 However, interference with the strut artefact limits the determination of dense calcium and necrotic core in the plaque behind the stent.
Intravascular Ultrasound Echogenicity
IVUS echogenicity analysis is a technique that calculates the relative fraction of hypoechogenic versus hyperechogenic tissue volumes. Tissue components are classified as either hypoechogenic or hyperechogenic using the mean grey value of the adventitia.19–21 Degradation of BRS can be determined by comparing the hyperechogenicity values directly after implantation with follow-up. Immediately after implantation the stented region typically shows increased hyperechogenicity compared with pre-implantation, suggesting the introduction of the stent. Over time, this hyperechogenicity diminishes, suggesting resorption of the BRS.22Figure 1 shows cross-sections of a poly-l-lactic-acid (PLLA) and a metal BRS over time using IVUS and IVUS-echogenicity analysis. In case of dramatic changes in the plaque morphology, e.g. high neointimal burden or development of calcified plaque, the e chogenecity analysis could however be affected. OCT is not affected by the presence of calcium and because of its ability to longitudinally assess BRS, OCT could be a useful technique for BRS analysis.
Optical Coherence Tomography
OCT is a high-resolution intracoronary imaging technique that uses a near-infrared light source (approximately 1,300 nanometres [nm] wavelength) to create the image. The axial resolution is about 10–15 micrometres, one order of magnitude higher than that of conventional IVUS, allowing for more detailed imaging. As a result of its high resolution, OCT can visualise the presence of atherosclerotic plaque, characterise the structure and extent of coronary plaque and quantify lumen dimensions as well as the extent of lumen narrowing in great detail.23,24 However, OCT has a limited penetration depth of 1–2 millimetres (mm), which is dependent on the penetration depth of the incident light beam into the vessel wall – the depth of penetration is greatest for fibrous tissue, least for red thrombi, and intermediate for calcified and lipid-rich tissue.25,26 Despite the limited penetration depth, the high resolution of OCT enables imaging of the length of atherosclerotic plaque and therefore potentially makes it possible to select the most appropriate stent length, landing zones and reference lumen dimensions that allow for optimal stent diameter selection. OCT also enables the visualisation of stent expansion, permitting safe and predictable post-dilatation in case of stent under-expansion. Furthermore, OCT is able to quantify the interaction of the stent with the vessel wall by its ability to determine stent apposition, the degree of malapposition, prolapse and intra-stent or edge dissections.27–29 Several studies have been performed to evaluate the reliability of OCT for the assessment of atherosclerotic plaque and stents, by assessing variability within and reproducibility of the data. An OCT reproducibility study of quantitative stent analysis showed that the relative difference for lumen area, stent area, tissue coverage area, tissue coverage thickness and strut coverage was around 1 % for the inter- and intra-observer reproducibility.23 For the lumen area on frame level, low intra- and inter-observer variability have been reported.30 Furthermore, a recently published study that evaluated pullbacks of stented coronary segments in time showed a very low per-frame and per-stent inter-study variability for mean lumen and stent area, suggesting that OCT is a reliable imaging tool for the assessment of repeated OCT examinations in longitudinal studies.31
Longitudinal evaluation of BRS is important for the quantification of strut degradation and the effect of the underlying plaque on the performance of BRS.32,33 The unique ability of OCT to reconstruct three-dimensional (3D) images allows for accurate evaluation of the interaction between the stent and the vessel wall, e.g. stent location, expansion and stent strut apposition.34 Jailed side branches have always been a source of concern because of the possible occurrence of thrombosis, alteration of shear stress, embolisation or endothelial coverage. Moreover, using 3D OCT, a classification based on the number of compartments delineated by the struts and the geometric configuration in front of the ostium has been developed.10 OCT potentially allows for selecting the most appropriate stent length, landing zones and reference lumen dimensions that allow for optimal stent diameter selection. Another light-based intracoronary imaging technique – NIRS – might, however, provide a better possibility to select the appropriate stent length and landing zones by its ability to assess the presence and location of lipid-core plaques with high accuracy.35,36
Near-infrared Spectroscopy
This technique uses near-infrared light of wavelengths from 800 to 2,500 nm to identify specifically the presence of lipid core plaques (LCP).35,36 The primary presentation of the data is the chemogram, a plot of NIRS values obtained during a rotational pullback within the coronary artery, that assigns the probability of the presence of LCP (yellow=high, red=low). The lipid core burden index (LCBI) score summarises the fraction of LCP in the imaged section of the coronary vessel on a scale of 0–1,000. Since NIR spectroscopy alone only provides chemical information, a combined LipiScan™ IVUS system (TVC Imaging system™; InfraReDx, Inc) has been developed to provide structural information as well. This new imaging catheter combines the advantages of NIRS and IVUS, and enables the determination of the structure of a plaque simultaneously with its chemical composition.37–40 A series of cases that highlight the potential clinical applications of the TVC Imaging system has been reported recently, showing that it may enhance detection of culprit and non-culprit lesions having architectural and compositional features of vulnerable plaque.41 Moreover, TVC imaging potentially helps in deciding on the correct stent length and to identify lesions at greater risk of distal embolisation during PCI.42 In studies evaluating BRS, the TVC system is mainly used for the evaluation of the change in LCBI from pre- to post-implantation and for the evaluation of the lipid content within the coverage of the stent at follow-up.43
Intracoronary Evaluation of Currently Available Bioresorbable Stent – Lessons Learned
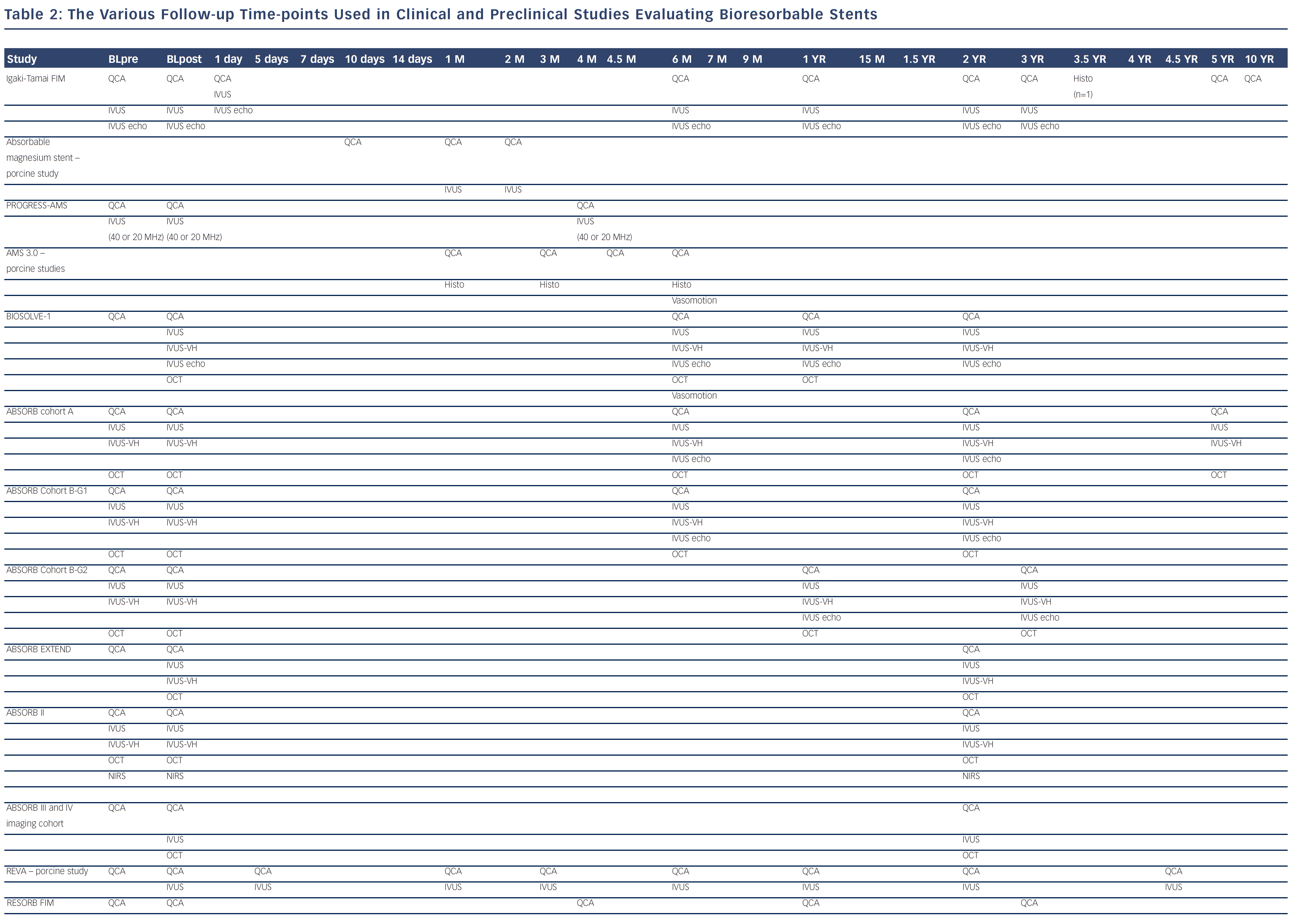
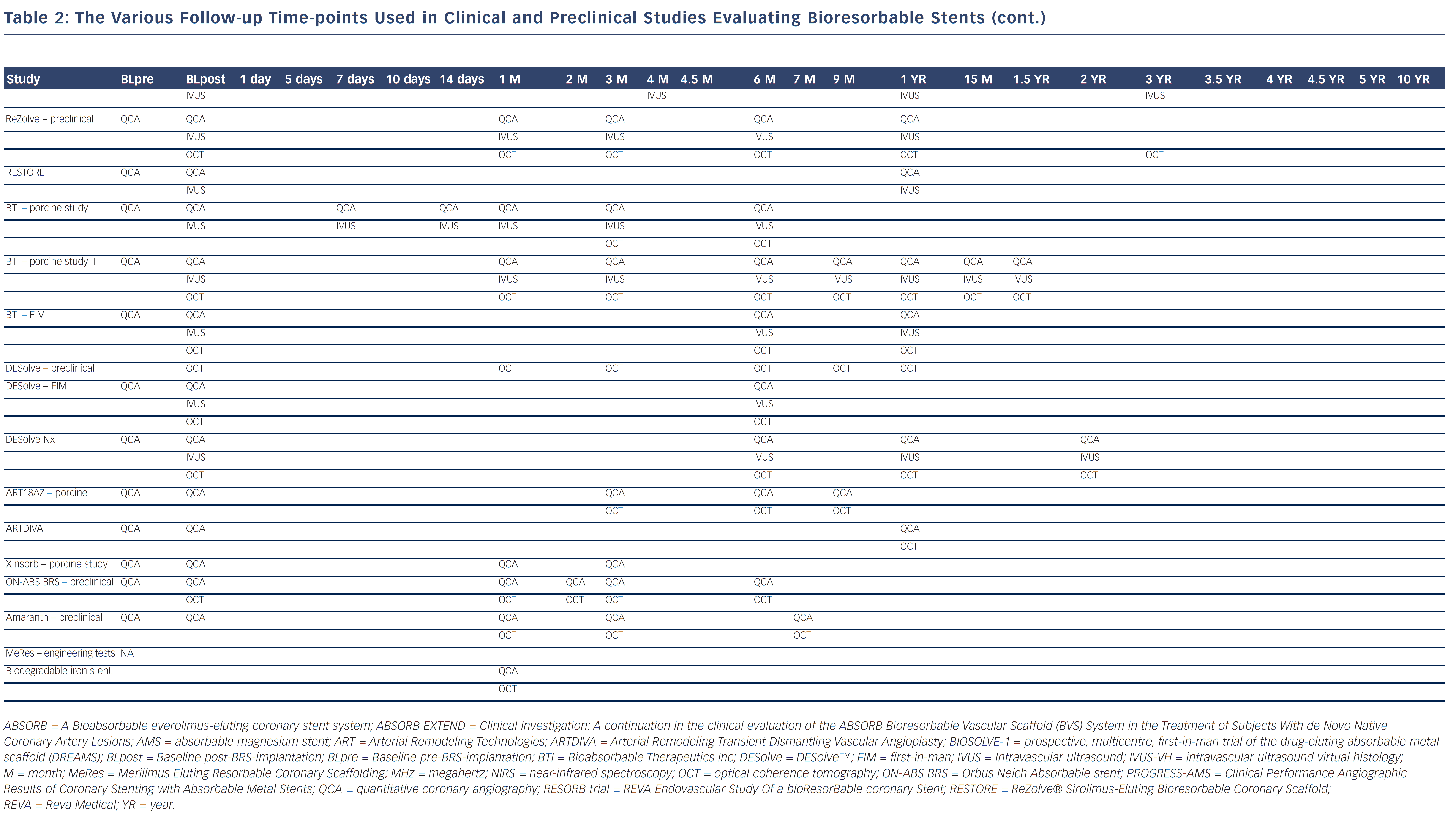
Bioresorbable technologies for stents were already investigated in the mid-1980s with the pioneering work at Duke University. Back then the first polylactic acid (PLA)-based bioabsorbable stent was a braided, self-expanding stent. Five years later the first balloon-expandable PLA stent was developed at Duke University.44 Until now, few different fully BRS have been tested in humans and animals. Table 1 shows the currently available BRS and their design. The design of BRS is important regarding the efficacy and safety, e.g. mechanical integrity and extent of re-endothelialisation, of the device. To accurately investigate the safety and efficacy of these BRS in vivo, longitudinal evaluation of their behaviour is mandatory and selection of the follow-up time-points should be performed carefully, taking the resorption and degradation pattern known from preclinincal studies in consideration. Table 2 shows studies evaluating BRS at specific time-points using different imaging techniques.
Igaki-Tamai® Stent (Kyoto Medical Planning Co Ltd, Kyoto, Japan)
The Igaki-Tamai BRS represents the first fully biodegradabable PLA-based coronary stent tested in humans in the late 1990s. This stent is self-expanding, requires storage and expansion at pre-specified temperatures and needs a larger guide catheter (8 French [Fr]). In the first report that described the Igaki-Tamai® stent, angiography and IVUS were used to evaluate the safety and efficacy of the stent up to six months. Acceptable restenosis and target lesion revascularisation (TLR) rates (both 6.7 %) and no deaths, myocardial infarction (MI) or coronary artery bypass grafts (CABG) were demonstrated. However, against expectation, the stent did not fully degrade six months after implantation and therefore clinical and imaging follow-up investigations were extended.11 Currently, imaging and clinical data of the first-in-man (FIM) Igaki-tamai is available up to 10 years demonstrating long-term safety, with similar major adverse cardiac event (MACE) rates to those of BMS (MACE free survival at 10-year 50 %) without stent recoil and vessel remodelling.19
Absorbable Magnesium Stent (Biotronik, Berlin, Germany)
The absorbable magnesium stent (AMS) is a magnesium-based balloon-expandable stent. A study in swine showed that this stent has a faster degradation time than PLA-based BRS, with a complete absorption of the stent within two months.45 The first evaluation of this BRS in humans was therefore performed in a prospective, non-randomised, multicentre clinical trial (PROGRESS-AMS) using angiography and IVUS (40 MHz; Boston Scientific, Natick, US or 20 MHz; Eagle Eye, Volcano, Rancho Cordova, US) at baseline and at four-month follow-up. Furthermore, clinical follow-up was performed at four, six and 12 months.46 The imaging results showed good scaffolding of the vessel, but a high event rate and in-stent late loss due to vessel recoil (55 %) and neointima formation (45 %).47 Therefore, a new device was developed including a prolonged scaffolding time and the anti-proliferative drug paclitaxel (Drug-Eluting Absorbable Metal Scaffold [DREAMS]; AMS 3.0). This paclitaxel-eluting AMS 3.0 was first evaluated in animal studies 48 and lead to a prospective, multicentre, FIM trial – the BIOSOLVE-1 (prospective, multicentre, first-in-man trial of the drug-eluting absorbable metal scaffold [DREAMS]) study. Angiographic follow-up was chosen at six and 12 months because of findings from animal trials that showed that the conversion of the magnesium to magnesium hydroxide is completed after six months and absorption to amorphous calcium apatite at 12 months. To capture all the potential late events and complications, clinical follow-up was planned up to 36 months.49 At six months, vascular restoration was achieved with the return of vasomotion50 and no substantial expansive or constrictive remodelling was observed.51 Furthermore, the absorption of the BRS was observed by IVUS-VH suggesting absorption by a reduction in dense calcium, by IVUS echogenicity suggesting absorption by a reduction in hyperechogenic tissue components, and by OCT confirming that the visibility of the stent began to fade.50 The recently published one-year results showed feasibility and safety of the AMS with 100 % procedural and device success, a TLR rate of 7 % and no cardiac death or scaffold thrombosis.9 The second-generation AMS 4.0 underwent additional design changes and elutes sirolimus. Preclinical swine data show lower inflammation and injury scores, and a higher endothelialisation score on histopathology at 28 and 56 days. BIOSOLVE-II planning is underway and will commence later in 2013.52
Abbott Vascular Bioresorbable Stent (Bioresorbable Vascular Scaffold; Abbott Vascular, Santa Clara, Ca, US)
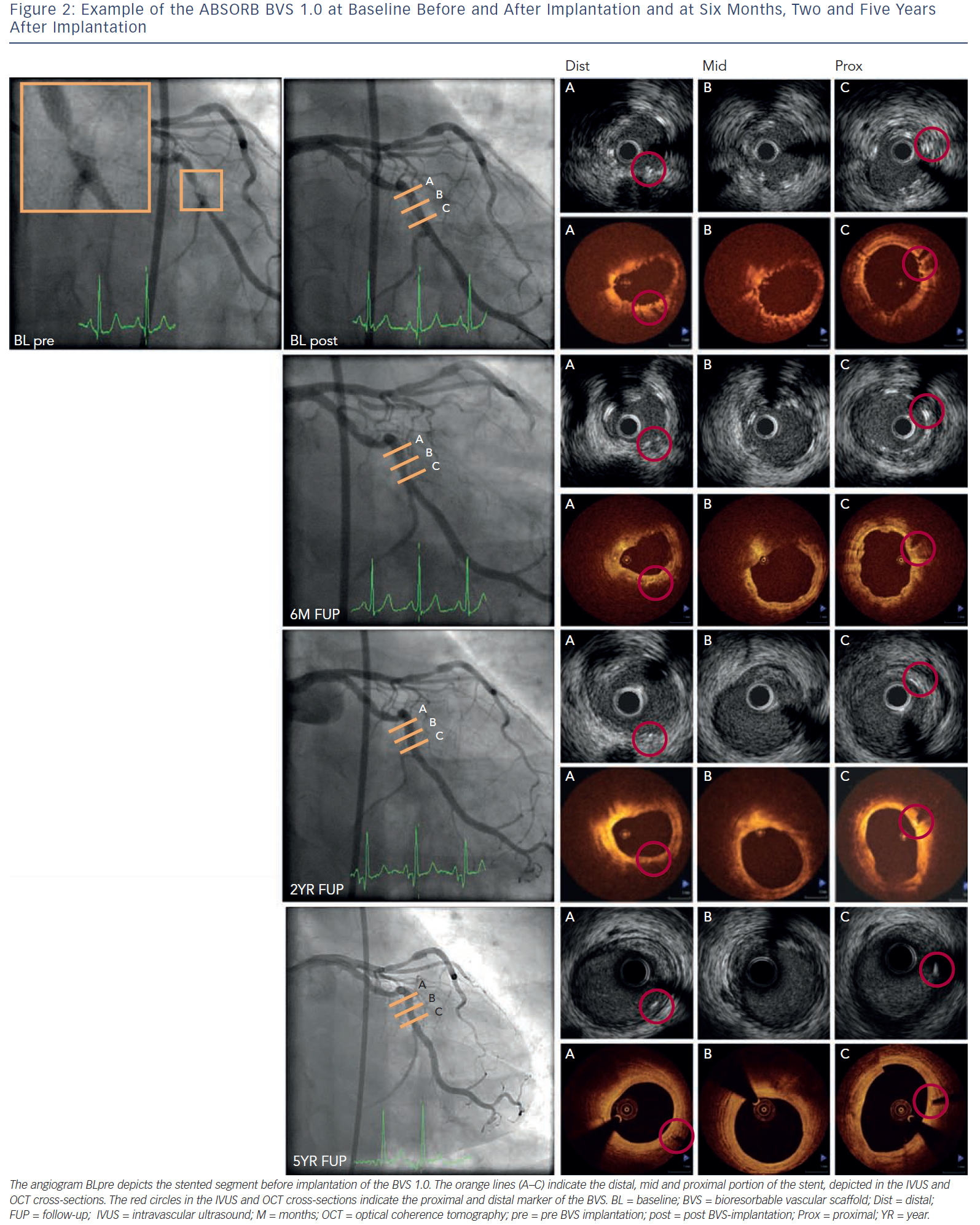
This BRS is a PLLA-based stent that elutes the antiproliferative drug everolimus and has the vastest imaging experience. The in vivo polymer degradation profile of the stent was established in a porcine coronary artery model and showed a mass loss of 30 % at 12 months with further reduction to 60 % mass loss by 18 months.53 The FIM evaluation of the Abbott vascular BRS (bioresorbable vascular scaffold [BVS] 1.0; Abbott Vascular, Santa Clara, Ca, US) was performed in the ABSORB (A Bioabsorbable everolimus-eluting coronary stent system) Cohort A study with imaging planned at six months and clinical follow-up at 12 months.20 Greyscale IVUS and OCT showed scaffold shrinkage between baseline and six months and therefore design changes were made. Invasive imaging evaluation was extended up to two years and clinical follow-up up to five years to capture potential late events.33,53 Between six months and two years, angiography showed a similar in-stent late lumen loss (LLL) and IVUS and OCT showed an increased minimum lumen area (MLA) and mean lumen area. After evaluation of the imaging results at two years, an extra invasive imaging evaluation was planned at five years and demonstrated an increased MLA and reference vessel diameter inside the scaffold. No MACE occurred and no scaffold thrombosis was seen, indicating late luminal enlargement and safety of the scaffold up to five years.54–56Figure 2 shows an example of the BVS 1.0 before and after implantation, at six months, two and five years.
The new generation Abbott vascular BRS (BVS 1.1) is studied in the ABSORB Cohort B trial, consisting of two arms: cohort B1, including 45 patients that underwent invasive (quantitative coronary angiography [QCA], IVUS, IVUS-VH, IVUS echogenicity and OCT) follow-up assessment at six and 24 months and cohort B2, including 56 patients that underwent the same invasive imaging assessment at 12 and 36 months. These two arms were chosen because, despite the prolonged scaffolding time at six-month follow-up of the BVS 1.1, it remained uncertain whether this favourable result at six months would persist in humans or whether a delayed inflammatory response accompanied by late recoil and neointimal hyperplasia would occur mid-term at 12 months. In the cohort B1 group angiography and IVUS showed a slight decrease in lumen area up to two years.21,57 OCT showed a similar lumen area decrease from baseline to six months, with no further changes at two years and an increased scaffold area up to two years. The IVUS-VH results showed a non-significant reduction in dense calcium up to six months.57 In the cohort B2 group, LLL at one-year was the same as found at two years in the cohort B1 group.21,58 The return of vasomotion, assessed by challenging the scaffolded segments with incremental doses of acetylcholine and nitrates, was observed at 12 months and even more pronounced at 24 months, suggesting a link between scaffold degradation and restoration of vasomotion in the treated segments.58 IVUS echogenicity analysis showed that at the expected time of total degradation and bioresorption, two years after implantation, the acoustic signals exhibited only a little evidence of polymer residues in both revisions of the BVS.20,21
In the currently ongoing ABSORB EXTEND (Clinical Investigation: A continuation in the clinical evaluation of the ABSORB Bioresorbable Vascular Scaffold (BVS) System in the Treatment of Subjects With de Novo Native Coronary Artery Lesions) trial, a non-randomised, single-arm, continued access trial, patients are treated with a 3.0 or 2.5 x 18 mm, or 3.0 x 28 mm BVS 1.1 to continue assessment of the safety and performance of this scaffold. A consistency in outcomes between ABSORB EXTEND and ABSORB Cohort B is seen.59 In the ABSORB II trial, a prospective, randomised control trial that aims to compare the safety and efficacy of the BVS 1.1 versus Xience PRIME, patients with stable angina and single or two vessel disease will be included and randomised on a 2:1 basis to BVS 1.1 and Xience PRIME stent implantation. IVUS-VH and NIRS will be used in addition to angiography and IVUS, pre- and post-procedure and at two-year follow-up to assess vessel geometrical and compositional changes over time as well as signs of scaffold resorption.43,60
Recently presented is the ABSORB III and IV pivotal clinical trial programme, a clinical programme consisting of two integrated randomised trials designed to achieve approval of ABSORB in the US and to demonstrate the superiority of ABSORB compared with DES. In the ABSORB III trial patients will be randomised on a 2:1 basis to ABSORB or Xience, in the ABSORB IV trial patients will be randomised 1:1 to ABSORB or Xience and a subgroup of these patients will be randomised to angiography, IVUS, OCT and vasomotion analysis.61
REVA (REVA Medical Inc, San Diego, US)
The first generation REVA BRS is a non-drug-eluting scaffold composed of tyrosine-derived polycarbonate, with a unique radio-opaque slide and lock mechanism to allow for visualisation on X-ray. The first REVA BRS were tested in Yucatan mini-swine immediately after implantation, at five days, three and six months and one, 1.5, two and 4.5 years after implantation using in vivo QCA, IVUS and OCT.62 The first-time evaluation of the REVA BRS in humans was performed in the RESORB (REVA Endovascular Study Of a bioResorBable coronary Stent) trial directly after implantation, at four, 12 and 36 months and showed a minimum lumen diameter increase post-procedure and the absence of late recoil was suggested because of an unchanged external elastic lamina at follow-up. However, there was a higher TLR rate than anticipated between four and six months, and therefore design changes were made.63 The second generation sirolimus-eluting REVA BRS was first tested preclinically in swine using QCA, IVUS and OCT directly after placement, at one, three, six and 12 months.62 Thereafter, the ReZolve® Sirolimus-Eluting Bioresorbable Coronary Scaffold (RESTORE) clinical trial was initiated.64 At six-month follow-up two MACE were reported, one TLR for focal in-stents restenosis, and one TLR directly related to protocol deviation at implant. Twelve months data will be presented at EuroPCR in May 2013. Furthermore, the RESTORE II trial has commenced investigating the sheathless ReZolve2 scaffold system, which will provide necessary data for the European Conformity (CE) marking.65
Bioabsorbable Therapeutic Inc (Menlo Park, CA, US)
The Bioabsorbable Therapeutic Inc (BTI) BRS is synthetised entirely from salicylic acid bioabsorbable polymer derivates and contains a top coat for sirolimus drug-elution. The first evaluation of the BTI was performed in domestic swine using QCA and IVUS post-implantation, at one and two weeks, and at one, three and six months after implantation. QCA showed good mechanical performance during placement and at follow-up a preserved apposition with maintained radial strength was observed using IVUS and OCT.66 Another evaluation of the BTI BRS in swine using QCA, IVUS and OCT directly after implantation, at one, three, six, nine, 12, 15 and 18 months demonstrated gradual degradation of the device. After 15 months most of the struts were not identified by OCT, probably because of replacement by neointimal tissue.67 The FIM study performed in 11 patients using QCA, IVUS and OCT post-implantation at six and 12 months showed acceptable safety and the absence of recoil. Insufficient neointimal suppression was however found, which was thought to be due to insufficient drug dosing on the surface area and a too fast drug release. New improvements are made with the second generation BTI that has improved manufacturing with a higher drug dose with slower release and an optimised stent pattern.67
DESolve (Elixir Medical, Sunnyvale, US)
The DESolve BRS is a PLA-based BRS with radial strength for over three months in which bioresorption occurs between one and two years as shown by OCT data from swine. In the FIM study, angiography, IVUS and OCT were performed post-implantation and at six-month follow-up, and showed no late scaffold recoil and low neointimal hyperplasia.68 The next evaluation of the DESolve BRS will be performed in the DESolve™ Nx study in which patients will be followed-up for five years. In a subset of patients QCA, IVUS and OCT will be performed at six, 12 and 24 months to provide more detailed information on the BRS and its degradation in time.68–70
ART18AZ (Arterial Remodeling Technologies, Noisy le Roi, France)
The ART18Z is a drug-free, flexible, bioresorbable polymer scaffold of which the polymer chains have no specific orientation resulting in more freedom to follow deformations and a supported overexpansion of 25 % without cracking or malapposition. Dismantling starts at three months with recovery of arterial function, and the resorption is expected within 18–24 months. The first evaluation of the Arterial Remodeling Technologies (ART) BRS was performed in porcine coronary arteries using QCA pre- and post-implantation at three, six and nine months and OCT at three, six and nine months. QCA and OCT analysis showed low acute recoil and low LLL at three and six months with a lumen area enlargement at nine months.71 The FIM evaluation, the Arterial Remodeling Transient Dismantling Vascular Angioplasty (ARTDIVA) trial, is a prospective, multicentre, open-labelled, single-group interventional investigation that aims to evaluate the safety of the ART BRS in the treatment of patients with single de novo native coronary artery lesions up to 12 months. The first impressions are excellent procedural performances and good apposition. To date, nine ART BRS have been implanted in patients and no MACE have been reported.72–74
Xinsorb (Shandong Huaan Biotechnology Co Ltd, China)
The Xinsorb BRS is a fully resorbable, balloon-expandable, PLLA stent coated with a thin layer containing sirolimus. The first evaluation was performed in a study comparing Excel™ DES (JW Medical System, Weihai, China) to Xinsorb DES. QCA analysis before, immediately after, and at one and three months after implantation suggests a successful prevention of elastic recoil and suppression of neointimal formation with a mildly delayed (1–3 months) re-endothelialisation of the stented vessel. Longer term follow-up is, however, required to validate the long-term efficacy of this Xinsorb BRS and before a FIM trial will be conducted, further preclinical examination will be performed.75
ON-ABS BRS (OrbusNeich, Fort Lauderdale, FL, US)
The OrbusNeich Absorbable (ON-ABS) BRS is composed of three distinct bioabsorbable polymer systems – Poly-D-lactide, PLLA and L-lactide-co-ε-caprolactone. This BRS is currently under preclinical evaluation using QCA and OCT before, directly after and at one, two, three and six months following implantation in swine.76 Preliminary OCT data immediately after implantation demonstrate a well apposed scaffold, no tissue prolapse, no broken struts and less than 2 % acute recoil.77 The FIM evaluation of this device is scheduled to start beginning 2014.78
Amaranth (Amaranth Medical Inc, Mountain View, CA, US)
The Amaranth BRS is a non-drug-eluting PLLA-based scaffold and is studied preclinically using QCA and OCT immediately after and at one, three and seven months after implantation. QCA showed minimal scaffold recoil, sustained structural integrity and a comparable radial strength up to seven months when compared with BMS. Neointimal thickness increased within 28 days and stabilised until 90 days. However, between 90 days and seven months the neointimal thickness seemed to decrease, suggesting late luminal enlargement. The FIM investigation started in 2013. Later in 2013 a drug-eluting version of this BRS will be tested in a porcine model.79
MeRes (Meril Life Science, India)
The MeRes (Merilimus Eluting Resorbable Coronary Scaffolding) BRS is another PLLA-based BRS, eluting sirolimus and coated with a special coating formulation that ensures the visibility of the stent under X-ray. The currently available results are from engineering tests that demonstrate a desirable performance. Preclinical tests in porcine coronary arteries are currently starting to understand the biological behaviour of the stent and the FIM clinical trials are expected to start in 2013.80
Lifetech Iron Bioresorbable Stents (Lifetech Scientific, Shenzhen, China)
This BRS is a biodegradable iron stent. A feasibility study of biodegradable nitriding iron stents was performed in coronary arteries of mini-swine in order to develop this stent. Eight iron stents were compared with eight vision stents (Abbott Vascular, CA, US) at four weeks after implantation using OCT. OCT data showed good biocompatibility with a similar neointimal proliferation in both stents. No thrombosis, inflammation and necrosis was observed in both groups and OCT showed 99 % neointimal coverage of the iron stents. Corrosion of the iron stents was observed without any signs of iron-related organ toxicity.81 However, not mentioned above, there are other BRS that are currently under development. These are the Sahajanand (Sahajanand medical technologies Pvt Ltd, India), Avatar (S3V; Vascular technologies Pvt LTd, India) and Zorion (Zorion Medical, IN, US). Detailed descriptions of these BRS are not yet publicly available.
Future Perspective on the Use of Intracoronary Imaging in the Bioresorbable Stent Era
The use of various imaging modalities is important to evaluate the different aspects of the degradation and behaviour of the stents. Co-registration of the different imaging modalities could, however, be challenging. A sub-study of the ABSORB cohort B1 compared QCA, IVUS and OCT findings and showed that OCT was most accurate in assessing stent length compared with nominal length (95 % confidence interval [CI] of the difference: -0.19; 0.37 and -0.15; 0.47 mm2). QCA consistently underestimated the length and IVUS showed low accuracy with several outliers and random variability. Within the three imaging modalities, poor agreement for MLA estimation was seen and no linear relation between any of the methods was demonstrated.82 Three-dimensional QCA allows for integration with IVUS or OCT and could therefore be used for accurate co-registration.83 Both IVUS and OCT correlate well with 3D QCA in assessing lumen size.84
Preclinical Study Considerations
Not all aspects regarding BRS can be addressed or answered in clinical studies. Therefore, well-established human-like models expressing coronary or peripheral arterial disease in which BRS can be implanted in the vascular bed they are intended for, are necessary. An easily accessible model is the farm bred swine fed a high-cholesterol but calorie-restricted diet. These swine show coronary artery disease (CAD) and a similar coronary anatomy to humans. They allow to study the development of atherosclerosis using intracoronary imaging, and to assess the longitudinal vascular response to bare metal and drug-eluting BRS in the presence of disease, which may exacerbate the vascular response. Moreover, the ability to obtain corresponding histological sections allows for the validation of BRS-behaviour, e.g. degradation and drug release, in the presence of coronary atherosclerosis.85 Furthermore, functional vascular assessment (vasomotion) can be performed both in vivo and in vitro and can be compared with corresponding histology.
Histological assessment of BRS is, however, challenging. Since BRS disappear or become flexible upon degradation, the utmost care needs to be taken in the preparation for histology to prevent post-mortem contraction and processing artefacts. The resulting changes in length and diameter of the segment of interest affect measurements and cross-correlation to imaging and could subsequently affect interpretation of the data. Depending on the chosen processing method, post-mortem changes cannot always be prevented but should be monitored and clearly described when reporting the data.
Conclusion
For the longitudinal evaluation of BRS, multiple intracoronary catheter-based imaging modalities are typically used. The main benefit of these intravascular imaging techniques is the ability to study the longitudinal changes in appearance and behaviour of BRS, which will aid in improving design and behaviour of these devices.