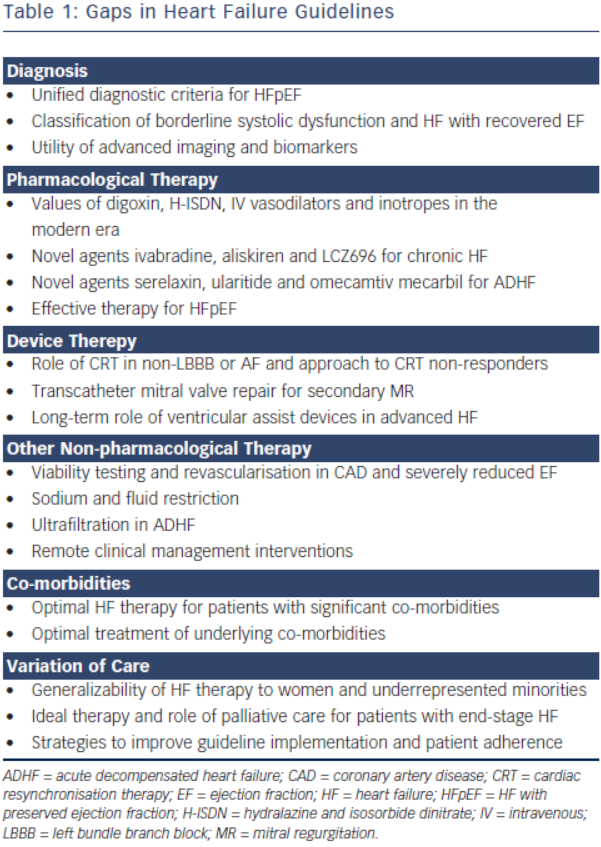
Heart failure (HF) remains a major public health problem resulting in substantial morbidity, mortality and healthcare expenditures globally. The European Society of Cardiology (ESC) 2012 Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure and the American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) 2013 Guideline for the Management of Heart Failure both provide comprehensive evidence-based recommendations in caring for patients with HF.1,2 Both guidelines use similar predefined scales for strength of recommendation and level of evidence for particular treatment options. The classes of recommendations range from Class I (where a given treatment is beneficial) to Class III (where a given treatment is not useful and in some cases may be harmful). The levels of evidence (LOE) range from Level A (where data have been derived from multiple randomised clinical trials [RCTs]) to Level C (where recommendations are based on consensus of expert opinions). The ACCF/AHA Guideline also emphasises the concept of optimal treatment, termed guideline-directed medical therapy (GDMT). Although guidelines do not substitute individual clinical judgment, improved adherence to HF guidelines translates to improved clinical outcomes in real world patients. It has been shown that each 10 % improvement in ACCF/AHA HF guideline recommended composite care was associated with a 13 % lower odds of 24-month mortality.3 However, there are still many aspects of HF care for which gaps remain in the evidence base, resulting in gaps in the guidelines. Only 19.5 % of the ACCF/AHA Guideline recommendations are considered well established by RCTs – 24 Level of Evidence A recommendations compared with 99 Level B or C. Similarly, only 34.4 % of the ESC Guideline recommendations are considered well established – 43 Level A compared with 82 Level B or C. Additionally, there are areas where new evidence has emerged but has not yet been incorporated into the guidelines. We aim to highlight these guideline gaps including areas that warrant further research, areas where data are conflicting and other areas where new data are forthcoming (see Table 1).
Gaps in Pharmacological Therapy
Substantial progress has been made in pharmacological therapy for HF with reduced ejection fraction (HFrEF) including angiotensin-converting enzyme inhibitors (ACEIs), beta-blockers and aldosterone antagonists, and novel agents continue to be developed. However, uncertainty remains with some of the oldest class of drugs. The vasodilator combination hydralazine and isosorbide dinitrate (H-ISDN) is the first therapy proven in a RCT to improve outcome in HFrEF. The initial Vasodilator-Heart Failure Trial 1 (V-HeFT I) showed 28 % mortality reduction compared with placebo, although this finding only reached borderline statistical significance (p=0.053).4 The follow-up V-HeFT II actually showed 28.2 % higher mortality with H-ISDN when compared with enalapril (p=0.016).5 Definitive mortality benefit of H-ISDN was finally established with the subsequent African-American Heart Failure Trial (A-HeFT) that enrolled self-identified African Americans with symptomatic HFrEF who were already on modern GDMT.6 The study terminated early as the H-ISDN arm showed 43 % decrease in all-cause mortality (p=0.01) and 33 % reduction in rate of hospitalisation (p=0.001) compared with placebo. However, the role of H-ISDN in non-African American patients with HFrEF in the modern era remains uncertain and warrants further research. The ESC Guideline currently gives H-ISDN an equivocal recommendation of Class IIb/LOE B in patients with HFrEF. The ACC/ AHAF Guideline recognises the differential treatment effect and gives H-ISDN Class I/LOE A in African Americans with HFrEF and Class IIa/ LOE B in other patients with HFrEF who cannot tolerate ACE inhibitor or angiotensin receptor blocker (ARB).
The use of digoxin, the oldest compound in cardiovascular medicine, declined after the disappointing Digitalis Investigation Group (DIG) trial, which showed a 28 % reduction in hospitalisations (p<0.001) but no difference in mortality.7,8 This trial, however, was done in an era where the current GDMT and device therapy were not commonly part of background therapy. Subsequent meta-analysis, retrospective studies and post-hoc analysis of more contemporary clinical databases have yielded conflicting conclusions, suggesting potential benefit as well as harm.9–13 Prospective RCT data would help clarify the role of digoxin in modern clinical practice in HFrEF with and without atrial fibrillation (AF).
The benefit of anticoagulation for stroke prevention in patients with AF is well established. However, in patients with very depressed ejection fraction (EF) who are at risk for intracardiac thrombi, anticoagulation has not been shown to be beneficial. Two RCTs of patients with HFrEF in sinus rhythm showed no clinical benefit and increased bleeding with warfarin when compared with aspirin.14,15 Given the improved safety and efficacy of the new oral anticoagulants (dabigatran, apixaban and rivaroxaban), these agents should be studied in subsets of patients with HFrEF in sinus rhythm that are at highest risk for thromboembolism.
Newer agents, ivabradine, aliskiren and LCZ696, are still establishing their roles in HF. The ESC Guideline gives ivabradine a Class IIa/LOE B recommendation in patients with symptomatic HFrEF and heart rate >70 beats per minute (BPM) based on the Systolic Heart Failure Treatment with the If Inhibitor Ivabradine Trial (SHIFT), which showed 26 % decrease in HF hospitalisations (p<0.0001) and a previous trial, which established its safety.16,17 In the US, ivabradine is still awaiting US Food and Drug Administration (FDA) approval and has not received formal recommendation in the ACCF/AHA Guideline. Aliskiren is a novel agent that targets the renin–angiotensin–aldosterone system (RAAS) to reduce blood pressure. The Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT) investigated aliskiren in patients with HFrEF and acute decompensated heart failure (ADHF). It showed increased rates of adverse effects, such as hyperkalaemia (relative risk [RR] 1.19 [0.98–1.46]), hypotension (RR 1.36 [1.07–1.72]) and renal failure (RR 1.37 [1.08–1.75]) in the aliskiren arm without benefit in mortality or hospitalisation.18 An ongoing RCT is investigating the role of aliskiren or aliskiren/enalapril combination in patients with chronic HFrEF (NCT00853658). Aliskiren has not received formal recommendation by the ACCF/AHA or ESC guidelines and its role in HF appears uncertain. In contrast to this is LCZ696, a novel dual-acting angiotensin receptor–neprilysin inhibitor (ARNI), which has the potential to shift the foundation of HFrEF therapy. The recent Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial was stopped early with LCZ696 demonstrating significant reduction in all-cause mortality (hazard ratio [HR] 0.84, 95 % confidence interval [CI] 0.76–0.93) and HF hospitalisations (HR 0.79, 95 % CI 0.71–0.89) as well as improvement in quality of life compared with enalapril.19 Based on these findings, we anticipate expedited approval of LCZ696 by both US and European regulatory agencies, and the addition of ARNI in the next ESC and ACCF/AHA Guideline update.
Gaps in Device Therapy
Cardiac resynchronisation therapy (CRT) has played an important role in the past decade in decreasing hospitalisations and increasing survival of patients with HFrEF. Though benefits are clear for symptomatic patients in sinus rhythm with typical left bundle branch block (LBBB) (particularly with QRS width >150 ms), there are some populations where data are equivocal.20 Post-hoc analysis of the major CRT trials showed no significant benefit in subgroups with non-LBBB morphology or subgroups with QRS duration <150 msec.21,22 Results are pending of the recently completed Pacing Affects Cardiovascular Endpoints in Patients with Right Bundle-Branch Block (PACE-RBBB) trial, which is evaluating whether univentricular right ventricular (RV) pacing can restore synchronisation in patients with right bundle branch block (RBBB) (NCT01169493). The benefits of CRT are also unclear in patients with AF where efficient CRT delivery is compromised by underlying conduction. Thus these patients have been excluded from most major CRT trials.23 In the Resynchronization–Defibrillation for Ambulatory Heart Failure Trial (RAFT) that evaluated CRT in patients with mild-to-moderate HF, subsets of patients with permanent AF had no clinical benefit with CRT.24 An ongoing RCT is evaluating the strategy of atrioventricular junction ablation to increase CRT response in patients with permanent AF (NCT01522898). Finally, although up to 30–45 % of CRT-implanted patients receive little benefit, the management of these CRT non-responders remains controversial.25 A meta-analysis suggests small improvement in left ventricular ejection fraction (LVEF) with CRT optimisation procedures, but it is unclear whether this would translate into hard outcomes, and the ideal optimisation protocol remains undefined.26
Device-based clinical management interventions for HF have shown mixed results. Previous trials of thoracic impedance monitoring and remote monitoring systems have failed to show improvement in outcomes.27,28 More recently, however, the Influence of Home Monitoring on Mortality and Morbidity in Heart Failure Patients with Impaired Left Ventricular Function (IN-TIME) trial showed that an implantable cardioverter defibrillator (ICD)-based telemonitoring system dramatically reduced mortality when compared with standard care (HR 0.37, 95 % CI 0.16–0.83).29 Another device, an implantable pulmonary artery pressure monitor, was shown in the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Functional Class III Heart Failure Patients (CHAMPION) trial to decrease HF hospitalisation (HR 0.72, 95 % CI 0.60–0.85).30 However, in both these positive trials the contribution of additional patient–physician interaction on outcomes cannot be underestimated. Notably there was a delay in FDA approval of the pulmonary artery pressure monitoring device over question of potential bias in preferential support of treatment group.31 Neither the ACCF/AHA nor the ESC Guideline endorses a remote monitoring strategy.
From a structural standpoint, new data about transcatheter mitral valve repair is encouraging. Secondary mitral regurgitation (MR) is a common consequence of left ventricular (LV) enlargement and dysfunction, but surgical repair has not been proven to be superior to medical therapy for functional MR.32 Two recent non-randomised trials reported results of transcatheter mitral valve repair MitraClip in patients with severe MR who were deemed too high-risk for surgery.33,34 In both studies more than 70 % of patients had functional MR. After mitral valve repair using MitraClip device, patients experienced improved clinical symptoms, decreased LV dimensions, and in one of the trials decreased mortality compared with a propensity matched cohort. Further prospective controlled trials are ongoing to define transcatheter mitral valve repair’s role in patients with symptomatic functional MR (NCT01626079 and NCT01772108). Pending results from these RCTs, the ACCF/AHA Guideline gives transcatheter mitral valve repair for functional MR an ambivalent Class IIb/LOE B recommendation, and the ESC Guideline does not give a specific class of recommendation about this topic.
Gaps in Non-pharmacological Therapy
Coronary artery disease (CAD) is the most common aetiology of HFrEF.35 While revascularisation with concurrent use of viability studies in severe ischaemic cardiomyopathy is logically sound, recent studies have challenged this dictum. The Surgical Treatment for Ischemic Heart Failure (STICH) trial showed no mortality benefit with coronary artery bypass grafting (CABG) when compared to medical therapy in patients with EF <35 %.36 Though notably, after taking into account patient crossover from the medical therapy arm, the ‘as-treated’ analysis showed decreased mortality with CABG (HR 0.70, 95 % CI 0.58–0.84). An imaging substudy of STICH showed that viability assessment by single-photon emission computed tomography (SPECT) or dobutamine echocardiography did not identify patients who would benefit from CABG.7 Cardiac magnetic resonance and position emission tomography imaging promise improved sensitivities and specificities in identifying viable myocardium, but their impact on clinical outcomes has not been rigorously tested.38 Thus the roles of viability testing and revascularisation in patients with CAD and severely reduced EF remain debatable. The ESC Guideline gives a viability testing Class IIa/LOE C recommendation and recommends against revascularisation in patients without viable myocardium (Class III/LOE C). The ACCF/AHA Guideline gives viability testing and revascularisation in patients with LVEF <35 % a Class IIa/ LOE B recommendation.
Though sodium and fluid restriction in patients with HF appears intuitive, its role is controversial. Even though sodium restriction is endorsed by many guidelines, small RCTs have shown worse neurohormonal profiles and increase in HF admissions for patients with HFrEF assigned to low-sodium diet.39–41 Similarly, other small trials have shown no significant benefit with fluid restriction in patients with HF.42,43 More recently, one RCT showed New York Heart Association (NYHA) class improvement in patients with chronic HF randomised to modest sodium and fluid restriction,44 while another RCT showed no clinical benefit with aggressive sodium and fluid restriction in hospitalised patients with ADHF.45 Despite conflicting data, the ACCF/ AHA Guideline gives sodium restriction and fluid restriction Class IIa/ LOE C recommendation, and the ESC Guideline gives only a general recommendation supporting sodium restriction and fluid restriction for symptomatic HF. Well-powered outcome trials are needed. Given the complexity of sodium and fluid homeostasis, perhaps the answer may be individualised targets based on the patient’s clinical status.
Other non-pharmacological interventions, such as self-management counselling, telephone support and home visitation have been advocated. However, there is no definitive evidence supporting an individual approach.46 While intensive multidisciplinary programmes have been found to reduce mortality and hospitalisation, the resources required to maintain this strategy have limited its ability to reach a wide spectrum of patients.47
Gaps in Acute Heart Failure Therapy
Despite significant advances in understanding the pathophysiology of HF, treatment of ADHF has changed little in the past decade. The mainstays of parenteral pharmacological treatments, such as diuretics, vasodilators and positive inotropes, improve haemodynamics but have not been shown to improve outcomes.48 The optimal diuretic regimen remains at the discretion of the clinician as the Diuretic Optimization Strategies Evaluation (DOSE) trial did not show clear benefits for low-dose or high-dose diuretics and bolus or continuous infusion.49 Contemporary trial of a common clinical practice, low-dose dopamine in ADHF, failed to show clinical benefits in the Renal Optimization Strategies Evaluation (ROSE) trial.50 Though intravenous nitrates and nitroprusside are widely used in practice, data demonstrating their safety and efficacy are sparse. The vasodilator nesiritide was widely used based on improvement in dyspnoea from the Vasodilation in the Management of Acute Congestive Heart Failure (VMAC) trial, but it fell out of favour after safety concerns were raised.51 Confirmatory trials demonstrated safety but also no significant clinical benefits.50,52 Ironically, given the number of trials, nesiritide has one of the largest bodies of evidence demonstrating safety compared with other pharmacological therapies for ADHF. Novel agents for ADHF that improve outcomes are urgently needed. The most promising of these is serelaxin, a peptide hormone with vasodilatory effect. In the Relaxin in Acute Heart Failure (RELAX-AHF) trial, serelaxin significantly reduced the primary endpoint of dyspnoea in patients with both HFrEF and HF with preserved ejection fraction (HFpEF).53,54 Unexpectedly there was a large reduction in the non-predefined endpoint of mortality (HR 0.63, 95 % CI 0.42–0.93), and a larger trial is looking to confirm this finding (NCT01870778). Another phase III trial is ongoing to evaluate ularitide, a synthetic natriuretic peptide, in ADHF (NCT01661634). Omecamtiv mecarbil, a novel inotrope-like agent, is awaiting phase III trial though phase II did not achieve the primary endpoint of reducing dyspnoea.55
From a non-pharmacological standpoint, contemporary ultrafiltration devices used for rapid fluid removal promised rapid decongestion in treatment of ADHF. The Ultrafiltration versus intravenous Diuretics for Patients Hospitalized for Acute Decompensated Congestive Heart Failure (UNLOAD) and Continuous Ultrafiltration for Congestive Heart Failure (CUORE) trials showed reduced readmission rates with ultrafiltration compared with diuretics (HR 0.56, 95 % CI 0.28–0.51 and HR 0.14, 95 % CI 0.04–0.48, respectively).56,57 However, the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial evaluating patients with ADHF and renal dysfunction showed only excess adverse events in the ultrafiltration group (72 versus 57 %, p=0.03), driven by worsened renal function, bleeding complications and intravenous catheter-related complications.58 Unfortunately, a larger trial to evaluate the role of ultrafiltration on readmissions for HF has been terminated due to patient recruitment challenges (NCT01474200). The ESC Guideline does not provide specific recommendation for ultrafiltration, and the ACCF/AHA Guideline gives it an equivocal Class IIB/LOE B recommendation. The ideal patient population who would benefit from ultrafiltration remains uncertain until more definitive data from larger trials are available.
Future clinical trials of therapies for ADHF should target HFpEF and HFrEF separately in addition to stratifying patients based on severity of decompensation and co-morbidities. Pressure to expand inclusion criteria to enrol enough patients to power studies for mortality benefits may ultimately dilute findings by increasing patient heterogeneity.
Gaps in Diagnosis and Treatment of Heart Failure with Preserved Ejection Fraction
The management of HFrEF has made substantial gains over the past three decades. In contrast, despite the high prevalence, mortality and morbidity of HFpEF, little progress has been made in establishing unified diagnostic criteria.59 The treatment of HFpEF remains largely opinion-based with little good evidence to guide therapy. Though promising in theory, trials of beta-blocker, ACEI, ARB, aldosterone antagonists, digoxin and phosphodiesterase type 5 (PDE-5) inhibitors have all shown largely disappointing results.60 Establishing broadly applicable therapies is hampered by the heterogeneity of the syndrome. LCZ696, discussed previously in HFrEF, was found to reduce N-terminal pro-brain natriuretic (NT-proBNP) and left atrial size in patients with HFpEF when compared with valsartan in a phase II RCT.61 The followup phase III trial powered to evaluate mortality has recently started recruiting patients (NCT01920711). Future studies should more distinctly subclassify different clinical phenotypes of HFpEF to target the dominant pathophysiology. And while mortality and hospitalisation are important clinical endpoints, they may be too insensitive for this heterogeneous population with multiple co-morbidities. There should be more focus on using health-related quality of life and other measures of health status as part of clinical trial endpoints to elicit meaningful results.
Additional ambiguity is seen in the intermediate group with EF between 40 and 50 %. These patients are often treated with therapy recommended for patients with HFrEF despite being underrepresented or excluded from most HFrEF trials.1 Finally, patients with a history of HFrEF where EF have recovered represent another subset where little is known about the natural history and prognosis. They likely represent a distinct phenotype in the spectrum of HFrEF and HFpEF and need further characterisation to determine the need for continued therapies.62
Gaps in Treatment of Co-morbidities
Common non-cardiac co-morbidities, such as anaemia, lung disease, kidney disease, diabetes and depression, likely play important roles in progression of HF and may interfere with diagnosis and therapy. Anaemia and chronic obstructive pulmonary disease (COPD) may confound the diagnosis of worsening HF. Depression may interfere with a patient’s ability to self-manage. Kidney disease often limits the use of ACEI/ARB, and severe lung disease may limit the use of beta-blocker. Frailty, cancer, gout, obesity and other co-morbidities may also directly affect HF therapy. The long-term safety and efficacy of many treatments for these co-morbidities in patients with HF are unknown. Moreover, as HF trials commonly exclude patients with significant co-morbidities, it is not clear whether GDMT have differential effects in these particular patients.63
Chronic obstructive pulmonary disease (COPD) can co-exist and confound the diagnosis of HF. Unfortunately, patients with severe COPD are often excluded from HF trials, so data are limited in this population.64 Despite concern about beta-blockers exacerbating COPD, it has been shown that even non-selective beta-blockers, such as carvedilol, are not associated with worse outcomes in patients with chronic HF and COPD.65 However, in the setting of an acute COPD exacerbation, the role of beta-blockers remains unknown. Additionally, the use of beta-2 agonist bronchodilators have been implicated in worsening HF, though this finding is limited by the observational nature of the data.66
Anaemia is a common finding in patients with HF and is independently associated with increased mortality risk.67 However, It is unclear whether anaemia is simply a marker of disease severity or a direct mediator of poor outcomes. Reduction of Events by Darbepoetin Alfa in Heart Failure (RED-HF), the largest RCT to evaluate erythropoiesis-stimulating agents in patients with HFrEF and anaemia, showed no difference in death or HF hospitalisation but increased thromboembolic events in the darbepoetin alfa group (13.5 % versus 10.0 %, p=0.009).68 In patients with HF and iron deficiency, however, the Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial showed that Intravenous (IV) ferric carboxymaltose improved NYHA class, six-minute walk distance and quality of life.69 A multicentre RCT evaluating oral iron in patients with HFrEF and iron deficiency is expected to start soon (NCT02188784).
Diabetes mellitus (DM) is highly associated with poor clinical status in patients with HF.70 The interaction between these two clinical syndromes is complex, and patients with DM have been shown to respond differently to HFrEF therapy compared with non-diabetics.71 From a DM therapy standpoint, while thiazolidinedione has clearly been shown to increase HF, the safety of newer therapies for DM – glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase 4 (DPP-4) inhibitors and sodium/glucose cotransporter 2 (SGLT-2) inhibitors – are unknown for patients with HF. Even insulin, an established treatment, has been associated with higher mortality in patients with advanced HF, though this may be more related to severity of diabetes.72
Chronic kidney disease (CKD) and the associated cardiorenal syndrome portend poorer prognosis and significantly impact management of HF patients.73 Significant renal dysfunction may preclude the use of ACEIs, ARBs and mineralocorticoids in patients with HFrEF. In addition, patients with advanced kidney disease (stage 4 and stage 5 CKD) and end-stage renal disease are frequently excluded from HF trials.74 In the setting of ADHF, no effective therapy for cardiorenal syndrome has been found, perhaps mirroring the lack of progress in ADHF care in the last decade.
Depression is also highly prevalent in patients with HF and independently predicts increased hospitalisation and mortality.75 However, there has been surprisingly little work done on defining the interaction between the two diseases and finding effective therapy. Although data have contested conventional wisdom that beta-blocker is associated with depression, beta-blocker’s effect on patients with concomitant HF and depression is unclear.76 While tricyclic antidepressants should be avoided in patients with HF due to known risks of QT interval prolongation and ventricular arrhythmia, the ideal antidepressant in HF patients is unknown. Sertraline Against Depression and Heart Disease in Chronic Heart Failure (SADHART-CHF), one of the few RCTs to date on this topic, found no benefit with sertraline in patients with HF and depression.77
Finally, pulmonary hypertension (PH) is a common complication of HF and is independently associated with poor prognosis.78 Unfortunately, there is no validated treatment for PH due to left heart disease. Perhaps due to patient heterogeneity, clinical trials have not shown benefits with prostanoids, endothelin-1 antagonists or guanylate cyclase stimulators in patients with HFrEF.79 For patients with HFpEF and PH, one small placebo-controlled trial showed that sildenafil increased exercise capacity and improved haemodynamic status.80 However a larger trial, Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure (RELAX), evaluated sildenafil in HFpEF and showed no difference in clinical outcomes compared to placebo.81 Ongoing trials are evaluating the role of sildenafil (NCT01616381) and tadalafil (NCT01910389) in patients with PH and HFrEF, and the role of riociguat in patients with PH and HFpEF (NCT01172756). Neither the ACCF/AHA nor the ESC Guideline specifically addresses patients with HF and PH.
Gaps in Variation of Heart Failure Care
In negative trials, the clinical heterogeneity of patient population is sometimes invoked as a reason why a therapy does not reach statistical significance. However, from a population perspective, most RCTs that form the current evidence base do not randomise a sufficient number of women and underrepresented minorities, thus limiting their generalizability. Women and various racial and ethnic groups have significant differences in aetiology of HF and response to treatment due to underlying biological differences and disparities in healthcare utilisation.82 Future trials should strive to enrol higher proportions of these underrepresented populations. Encouragingly, data from the Get With The Guidelines®-Heart Failure registry shows that a concerted national quality improvement programme can deliver equally effective care across racial and ethnic groups.83
In clinical trials, the impact of therapy on quality of life can sometimes be deemed less important than the primary endpoint of mortality. This issue is particularly relevant for very elderly patients or patients with poor long-term prognosis, where symptom control may be more valuable than mortality benefits. Additionally, palliative services and hospice remain underutilised in patients with advanced HF, especially when compared with patients with cancer.84 Determining the optimal timing and palliative care approach is difficult in HF because of the undulating course of disease and availability of advanced therapies for end-stage HF, such as heart transplantation and ventricular assist devices.
Conclusion
The economic burden of HF continues to grow and HF is one of the single most expensive and deadly healthcare problems. Additional clinical and comparative effectiveness research studies are urgently needed, along with development of new and innovative therapies. Significant gaps remain in the evidence base and guidelines for HF, particularly in the care of patients with HFpEF, patients with ADHF and patients with HF, and multiple co-morbidities. Other gaps in evidence that we did not address include the increasing use of ventricular assist devices, novel cardiac biomarkers and advanced cardiac imaging techniques. Along with encouraging novel devices and pharmacological therapies, it remains important to refine the roles of established therapies. When such evidence-based, guideline-directed therapies exist or emerge, every effort should be made to effectively implement these HF therapies to optimise care and outcomes.