Introduction
Advances in heart failure medical therapy over the past few decades have improved the prognosis of patients with this condition. Despite this, heart failure remains a significant burden to the medical system as the incidence of heart failure hospitalisation continues to rise.1 Diuretics have been a mainstay of therapy in heart failure to relieve congestion and improve symptoms. Despite the widespread use of diuretics, there is a lack of guidance on how to best titrate these medications in chronic use. Guidelines support the use of diuretics at the lowest clinically effective dose, but do not specify a diuretic strategy beyond that.2 Here we review the diuretics available for use in heart failure, potential mechanisms of diuretic resistance and ways to address this in the ambulatory setting, and review tools that have been developed with the goal to help guide diuretic use to treat patients with chronic heart failure.
Loop Diuretics
Loop diuretics remain the diuretic of choice for treating patients with heart failure.3 Furosemide, torsemide and bumetanide are the agents widely available for clinical use, with furosemide the predominant agent of the three. All three loop diuretics are available in oral formulation and are first absorbed in the gastrointestinal track. Once absorbed, the majority of the diuretic becomes protein bound in the vascular space, which in turn requires the drug to be transported into the nephron by organic anion transporters.4 Loop diuretics then travel to the ascending loop of Henle and inhibit the Na+/2Cl/K+ cotransporter to block reabsorption of sodium and chloride, resulting in natriuresis. Loop diuretics also induce renal prostaglandin synthesis, which results in renal and peripheral vascular smooth muscle relaxation and venodilation.5 The dose–response curve is sigmoidal, demonstrating that the drug concentration must reach a diuretic threshold to have an effect, and further diuresis above this threshold is achieved by increased frequency of administration rather than increased drug concentration.5
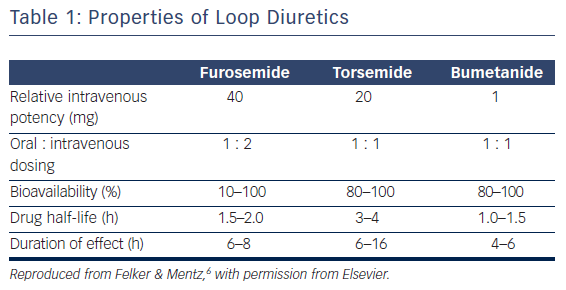
There are key pharmacokinetic differences between the loop diuretics (Table 1). Torsemide and bumetanide have an oral bioavailability of 80–100 %, while furosemide has a wide variant bioavailability of 10–100 %.6 Ingestion of food also has an effect on pharmacokinetics as it can decrease the maximum concentration of loop diuretics by one-half and increase the time to peak serum concentration by 30–60 min.7–9 The effect of food intake on the impairment of diuretic absorption is greater with furosemide and bumetanide, whereas torsemide’s bioavailability is relatively unchanged by food intake. The overall rate of absorption is also negatively affected when the patient is congested.10,11 In patients with chronic renal insufficiency, furosemide has been shown to have a variable dose response compared with a more consistent dose effect with bumetadine due to altered metabolism of furosemide in patients with kidney disease.12 With the oral formulations, furosemide has a half-life of 2 h, bumetanide has a half-life of 1 h, and torsemide has the longest half-life at 3.5 h.13 Furosemide is the most common loop diuretic prescribed but has a bioavailability that can be quite variable between similar patients as well as within the same patient during different disease states. This may be due to pharmacological factors inherent to furosemide and genetic differences between individuals as well.14,15
Despite the variable bioavailability, furosemide is commonly the first loop diuretic prescribed to patients with heart failure. However, there are few adequately powered or designed studies assessing the comparative effectiveness of these loop diuretics. The Torsemide in Chronic Heart Failure study is the largest study to date comparing furosemide to torsemide.16 The study found that after an average follow up period of 9 months in 1,377 patients, those in the torsemide group had a risk reduction in overall mortality, reduction in cardiac mortality and improvement in functional status. Unfortunately, the study was a non-randomised prospective cohort as it was a postmarket surveillance of safety, with surprisingly low use of beta-blockers and angiotensin converting enzyme inhibitors.16 Torsemide in Chronic Heart Failure was also an open label study, so the comparison between torsemide and furosemide was not without bias. Other smaller studies support the benefits of torsemide over furosemide, suggesting less heart failure-related hospital days but no differences in hospitalisations due to heart failure.17,18 Some of these plausible benefits might result from torsemide’s aldosterone antagonistic properties.19,20 Practically speaking, the use of torsemide is reserved in patients that have demonstrated some degree of diuretic resistance to furosemide. However, doubts remain regarding its impact on clinical outcomes. Bumatenide, on the other hand, is even less well studied and its impact on clinical outcomes in comparison to furosemide is still unclear.21
Loop Diuretic Resistance
A diminished response to loop diuretics, or diuretic resistance, can lead to an adverse clinical course and prolong hospital stays. Diuretic resistance occurs frequently, with data from the Prospective Randomized Amlodipine Survival Evaluation study reporting that up to 25 % of participants displayed diuretic resistance, defined as absolute diuretic dose above the population median dose.3 This is a common clinical scenario that requires an increase in diuretic dose for decongestion. Another metric of diuretic resistance is diuretic efficiency, which is the net fluid loss per mg of diuretic. It may be a more precise measurement of diuretic resistance then absolute diuretic dose and low diuretic efficiency has a stronger association with mortality than does absolute diuretic dose.22
Although loop diuretics have not demonstrated a mortality benefit in heart failure, evidence of diuretic resistance carries a poor prognosis with a higher predicted mortality and increased risk of readmissions for heart failure.22–25 Mechanisms to explain diuretic resistance are multifactorial. In a congested state, aspects of gut wall oedema, decreased splanchnic blood flow and decreased intestinal motility due to an increased sympathetic state all lead to a delayed time to maximum peak and decreased peak concentration of the drug.10 In patients with renal insufficiency, other organic acids such as blood urea nitrogen can compete with loop diuretics for transport by the organic anion transporters, with less drug therefore reaching the site of action. This decrease in drug concentration results in a failure to reach the diuretic threshold concentration needed for the drug to be effective.
Further changes in sodium handling in response to loop diuretics also contribute to diuretic resistance. During periods of decreased drug levels between diuretic doses there is a rebound in sodium reabsorption that has been termed a ‘post-diuretic effect’.26 A ‘braking phenomenon’ has also been described after chronic diuretic use due to renal adaptation. Hypertrophy of cells in the distal convoluted tubules, away from the site of action of loop diuretics, leads to increased efficiency of sodium reabsorption and decreases the effect of loop diuretics.25 And lastly, other drugs can contribute to diuretic resistance. Non-steroidal anti-inflammatory drugs are common over-the-counter medications that clinicians should ask their patients about as patients commonly view them as benign due to their availability but they can contribute to diuretic resistance as well as increased risk of hospitalisations.27
Diuretic resistance and decreased response to loop diuretics is, unfortunately, not uncommon in clinical practice. Guidelines do not dictate which loop diuretic to use, only to use the lowest dose possible to achieve the desired effect.2 As diuretic resistance becomes increasingly suspected due to a decreasing response to a stable dose of loop diuretic, the diuretic dose can be increased in an effort to achieve comparably efficacious natriuresis. Another option at this time can also be to change from one loop diuretic to another with the hope that better pharmacokinetics between the loop diuretics can achieve the desired effect. Changing from furosemide to torsemide is common due to more consistent bioavailability of torsemide and the longer half-life of torsemide can potentially counter the ‘post-diuretic’ effect seen with furosemide.
Ambulatory Intravenous Loop Diuretics
Another growing practice used to counter resistance to increasing oral doses of loop diuretic is to administer intravenous loop diuretics in the ambulatory setting. This strategy has been utilised by several centres to help reduce hospitalisations, specifically targeting those patients that would only require one or two doses of intravenous diuretic to achieve euvolemia. Preliminary reported experiences so far have demonstrated this as a safe and effective way to decongest hemodynamically stable patients and potentially reduce hospitalisations for heart failure and overall healthcare costs.28–30 These outpatient heart failure units may therefore be useful to address those stable patients who appear to have diuretic resistance not surmountable by oral doses and just need some decongestion in order to respond to oral doses again. Furthermore, these centers also present another opportunity to involve a multidisciplinary care team as these patients are being monitored for a few hours while being given intravenous diuretics.
Thiazide Diuretics
When higher doses of a loop diuretic or changing loop diuretics are not achieving adequate decongestion, adding non-loop diuretics is an additional strategy – commonly referred to as ‘sequential nephron blockade’. Thiazide diuretics and metolazone are frequently utilised with loop diuretics to achieve this sequential blockage as thiazide diuretics inhibit the Na+Cl– cotransporter in the distal convoluted tubule. This can counter sodium reabsorption that occurs with the increased sodium load being delivered to the distal convoluted tubule after loop diuretic administration, as well as counter the increased sodium transport capacity or ‘braking phenomenon’ that occurs with chronic loop diuretic use. Although the use of loop and thiazide diuretics is common clinical practice, the majority of the data on this combination diuretic use is limited to small observational trials or case studies.31 Despite this, the evidence in these studies supports the use of combination diuretic therapy in those on high dose loop diuretics demonstrating diuretic resistance.31 Common diuretics used to augment loop diuretics are metolazone, hydrochlorothiazide, chlorothiazide and bendroflumethiazide. Metolazone is commonly used for combination therapy, but no study thus far has demonstrated one thiazide-type drug as being superior to another in augmenting diuresis and this appears to be a class effect.31,32 This strategy does have risks, as a more robust diuresis with combination diuretic therapy can lead to electrolyte abnormalities including hypokalemia and, less commonly, hyponatremia and hypochloremia. The increase in decongestion can also worsen renal function if hypovolemia develops. Due to this, frequent monitoring of electrolytes and renal function is paramount when this strategy is used. When starting combination therapy in the outpatient setting, starting with a low dose of metolazone such as 2.5 mg (or equivalent dosing of another thiazide-type diuretic) two to three times a week and close monitoring to assess response and monitor for adverse events is warranted.31 Similar to loop diuretics, the concentration of thiazide diuretics must be adequate enough to cross a threshold for effectiveness. After the initial dose of thiazide diuretic, if there is not an augmentation in diuretic response, a higher dose of thiazide diuretic is then warranted. Although it is common clinical practice to administer the thiazide-type diuretic at least 30 min prior to the loop diuretic, evidence is lacking with regard to this practice as most studies administered the diuretics simultaneously.31
Mineralocorticoid Receptor Antagonists
Mineralocorticoid receptor antagonists (MRA), such as spironolactone and eplerenone, are common medications used in chronic heart failure to reduce adverse clinical outcomes.33–35 However, the doses commonly used and studied in these landmark trials have minimal diuretic effect, with their prognostic benefits likely resulting from their neurohormonal antagonism. In contrast, small studies have suggested that using doses in the range of 100–200 mg spironolactone daily may increase diuretic efficacy in those thought to have diuretic resistance and improve symptoms of congestion.36–38 The Aldosterone Targeted NeuroHormonal Combined with Natriuresis Therapy – Heart Failure study evaluated spironolactone 100 mg versus placebo in hospitalised patients with acute decompensated heart failure and did not find differences in their primary end point between NT pro B-type natriuretic peptide levels or their secondary outcome of dyspnea relief, clinical congestion, net urine output or weight loss between standard of care and high-dose spironolactone.39 Of note, there was no difference in the safety outcomes of incidence of hyperkalemia or change from baseline estimated glomerular filtration rates between the placebo and high-dose spironolactone groups at 96 h. Although this study demonstrated that high doses of spironolactone were reasonably well tolerated, the use of high-dose MRAs for treating diuretic resistance remains unclear. Although uncommon, the evolution of hyperkalemia remains a strong consideration with MRA use. Estimations suggest that the incidence rates in heart failure patients using MRAs range from 5 to 15 %.40–42
Vasopressin Antagonists
Vasopressin antagonists have also been considered for use in those with diuretic resistance. This class of drug blocks the effects of vasopressin on the aquaporin V2 receptor at the cortical collecting duct, leading to an increase in free water excretion or ‘aquaresis’. In the Efficacy of Vasopressin Antagonism Heart Failure: Outcome Study with Tolvaptan, the investigators evaluated the addition of tolvaptan 30 mg daily to intravenous loop diuretics in hospitalised patients for heart failure. There was an increase in weight reduction and lower discharge weight in the tolvaptan group, but no evidence of long-term benefit in morbidity or mortality.43 When tolvaptan was started in addition to chronic loop diuretic therapy in ambulatory patients with heart failure, a dose-dependent weight loss was observed early on, but this failed to translate into any long-term improvement in congestion with continued therapy.44,45 As a result of these disappointing findings in combination with two recent null clinical trials attempting to elucidate the use of tolvaptan as part of an additional strategy for the management of acute decompensated heart failure, the role of tolvaptan for the routine management of congestion remains in question.46,47
Strategies to Guide Diuretic Titration
Heart failure is the leading cause of hospitalisation in the US and Europe; from 2001 to 2009, more than 1 million patients were hospitalised with heart failure as the primary diagnosis, comprising 1–2 % of all hospitalisations.48 This, in addition to the high rate of readmission after heart failure hospitalisation, presents a significant burden to the healthcare system. Optimising diuretic therapy in heart failure patients is a challenging task. Not surprisingly, there has been an increase in research to investigate complementary tools to help clinicians monitor and adjust diuretics, as well as other therapies to avoid congestion and prevent subsequent hospitalisations. Here we review several recent advances, such as ambulatory hemodynamic monitoring both invasive and non-invasive, and the emergence of subcutaneous furosemide as a potential option for patients in the future.
Many patients with heart failure have implantable cardioverter devices in place, and these devices allow for the collection of additional clinical data such as heart rate variability, patient activity and arrhythmias. These devices are also capable of calculating intrathoracic impedance, which is a characteristic of the electrical current that can pass from the device case to the right ventricular electrode.49 As pulmonary congestion increases, the device senses a drop in electrical impedance. Not surprisingly, there is an inverse correlation between the intrathoracic impedance with both pulmonary capillary wedge pressure and net fluid loss during an acute hospitalisation.49 This information, in addition to other parameters measured from the device, may identify patients at risk for heart failure events, heart failure hospitalisations or 30-day rehospitalisations after discharge. However, whether thoracic impedance might guide diuretic titration and or impact doses in other heart failure therapies still has no clear impact on rehospitalisations or clinical outcomes.50–53
Direct pulmonary artery pressure device monitors have had promising results in the management of heart failure. The CardioMEMSTM device by St Jude Medical (Atlanta, GA, USA) is placed in the pulmonary artery during a right heart catheterisation and allows for measurement of pulmonary artery pressures. The CardioMEMSTM Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA class III Heart Failure Patients (CHAMPION) study evaluated this device. In that study, clinicians had access to the treatment group’s pulmonary artery pressure results but not the pressure results of the control group.54 Patients in the treatment group had a 28 % relative risk reduction in the primary end point of heart failure hospitalisations at 6 months, and over the entire study period of 15 months there was a 37 % relative risk reduction in heart failure hospitalisation in the treatment group.54 One caveat when interpreting these results is that the treatment group had additional clinical contact and support due to having a device implanted, which may have a had a confounding effect on clinical outcome.55 Post-marketing efficacy studies are ongoing, testing whether the benefits in the CardioMEMSTM device translate into improvement in real-world patient care.56 For example, a recent retrospective cohort study observed a reduction in heart failure hospitalisations in the 6 months after CardioMEMS implantation compared with the 6 months prior to implantation, supporting the results of the CHAMPION trial on the use of the device in addition to standard of care.57 At this point, however, the therapeutic role of the CardioMEMS device continues to evolve.
In contrast to invasive implantable monitors, non-invasive systems have been developed to assess pulmonary congestion and avoid acute decompensations. An electromagnetic energy-based technology (Remote Dielectric Sensing, ReDSTM) non-invasively measures the dielectric properties of tissues using low-power electromagnetic signals. This is performed non-invasively as patients wear a vest for a few minutes in which this measurement is recorded to give an indication of the fluid content in the lungs, a method that has been validated in prior studies using chest computed tomography as a comparator.58 A recent small study using 50 patients shows that in patients recently discharged for decompensated heart failure, outpatient therapy guided by the ReDSTM device results appears to decrease the rate of heart failure rehospitalisation.59 A larger multicentre trial is ongoing to further confirm these preliminary findings.60 Non-invasive lung impedance monitoring is also being investigated as a surrogate to monitor for pulmonary edema. A recent moderate-sized randomised control trial performed at two centres demonstrated that patients who had their heart failure therapies guided by lung impedance measurements at outpatient visits over 1 year had a reduction in hospitalisations for heart failure.61 If proven effective in improving clinical outcomes, these devices can potentially serve as another tool available for clinicians to monitor patients in the ambulatory setting and provide additional objective data to assist in titrating diuretics. However, non-invasive assessments, whether through measurement of electrical impedance, lung impedance or dielectric properties, with routine care have yet been shown to improve hard clinical outcomes in patients with heart failure. Nevertheless, these methods do provide additional insight that may guide chronic decongestion strategies.
Although furosemide has been used for decades, utilisation of a novel administration route by subcutaneous injection may be a viable alternative for patients with congestive heart failure. One early case series evaluated subcutaneous furosemide to a placebo injection of normal saline, with the group receiving subcutaneous furosemide demonstrating a higher amount of urine voided and higher urine sodium concentration.62 The subcutaneous furosemide group demonstrated a 30-min onset of diuresis and a duration of increased diuresis of be 3–4 h. Currently, this is an off-label use, with the limited published data available being from small case studies and uses in palliative care.63–65 The sc2WearTM Infusor, developed by scParmaceuticalsTM (Burlington, MA, USA), is a small pump that attaches to the body via an adhesive and delivers subcutaneous injections of medications. It is currently being investigated for the administration of subcutaneous furosemide in patients admitted for acute decompensated heart failure.66 The study will compare the strategy of early discharge with subcutaneous furosemide compared with usual care in a group of patients that have been stabilised but need further decongestion with intravenous diuretics. As a result, subcutaneous furosemide may be another option in the ambulatory setting for patients that become less responsive to escalating oral diuretic doses.
Conclusion
Diuretics play a pivotal role in the management of heart failure to decrease congestion and symptoms. This is made difficult by the development of diuretic resistance, which is sometimes insurmountable with the common practices of increased doses of diuretics or a change in the loop diuretic. Combination therapy is frequently employed, with thiazide-type diuretics being the most commonly used. Despite diuretics being used for decades in the treatment of heart failure, guidance on the best management strategy of heart failure is still lacking. Continued investigations into diuretics and fluid-management strategies will benefit our knowledge base on the use of these medications and hopefully improve clinical outcomes for our patients with heart failure.