Multivessel coronary artery disease (MVCAD) is defined by the presence of ≥50 % diameter stenosis of two or more epicardial coronary arteries. The presence of MVCAD indicates poorer prognosis and a significantly higher mortality than single-vessel disease. In MVCAD, revascularisation can be achieved by either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG).1,2 A comprehensive definition of the adequacy of myocardial revascularisation should take into account the size of the vessel, the angiographic and functional severity of the lesion, and the viability of the myocardial territory.3 Accordingly, anatomic and functional complete revascularisation (CR) are not always synonymous. Generally, the anatomic CR is defined by treatment of all ≥50 % stenosis in vessels of ≥1.5 mm diameter, whereas functional CR is defined by treatment of all lesions assessed as functionally relevant (with both invasive or non-invasive methods) in the presence of myocardial viability in the dependent territory, see Table 1.3
At present, only a few trials have been specifically designed to directly evaluate the adequacy of revascularisation. Current literature, mostly relying on meta-analyses of non-randomised observational studies,4,5 lists the adequacy of revascularisation among factors that should guide the choice of treatment strategy. In most patients with MVCAD, the main advantage of CABG over PCI seems to be conferred by the achievement of more extensive revascularisation. Most of the difference in terms of the benefit of CABG over PCI seems to derive from patients who undergo incomplete revascularisation (IR). As documented in a recent patient-level analysis, long-term mortality was similar between patients undergoing CR with either PCI or CABG, whereas it was significantly higher with IR after PCI than CABG.6
Although much less invasive than traditional CABG, PCI yields lower rates of CR in patients with multiple coronary lesions.7 IR can occasionally be implemented in addition to CABG in order to reduce complications, mainly when minimally invasive or off-pump surgery is attempted.8
The advantages of CR have emerged from long-term follow-up studies showing a direct relationship between the number of coronary segments treated and the reduction in cardiovascular events. IR reduces potential periprocedural complications, especially in high-risk patients; however, this comes at the price of a noticeable risk of future adverse cardiovascular events.3
Complete vs Incomplete Revascularisation in ST-elevation Myocardial Infarction
Available guidelines for myocardial revascularisation clearly state that the infarct-related artery (IRA) should be systematically treated during the initial intervention in patients presenting with ST-elevation MI (STEMI).1,2 Nevertheless, up to 50 % of these patients have MVCAD, with angiographic documentation of significant stenosis affecting a non-IRA. The presence of MVCAD in this context identifies a subgroup of patients with more than double the risk of death at 30 days than individuals in whom the IRA is the only diseased vessel.9
The PCI strategies available in patients with STEMI and MVCAD include: IRA-only primary PCI with medical management of non-culprit lesions in the absence of spontaneous angina or myocardial ischaemia on stress testing; MVCAD PCI at the time of primary PCI (ad hoc procedure); and primary PCI of the IRA followed by staged PCI of non-IRAs later during the index hospitalisation or soon after hospital discharge.1,2,10
Previous clinical practice guidelines recommended against PCI of non-culprit artery stenoses at the time of primary PCI in haemodynamically-stable patients with STEMI; however, there is now growing evidence that a strategy of multivessel PCI – either at the time of primary PCI or as a planned, staged procedure – may be beneficial and safe in selected patients with STEMI.1,10 A network meta-analysis has suggested that multivessel staged PCI may be associated with a better outcome than multivessel primary PCI,11 but such data are still insufficient to inform a recommendation with regard to the optimal timing of non-culprit vessel PCI.12–14 In fact, a more recent meta-analysis showed that CR at the index procedure or as a staged procedure – whether during hospitalisation or after discharge – was associated with a reduction in the risk of adverse events, although the effect was mostly due to a reduction in the risk of urgent revascularisation. There was no difference between various strategies in the risk of all-cause mortality and spontaneous reinfarction at a median of 25 months.15 The best revascularisation strategy of the non-culprit lesions is not, therefore, well established.16
Apart from the potential overestimation of the severity of non-IRA due to heightened vascular tone,17 major concerns when attempting CR in a STEMI derive from: the prolongation of the primary PCI procedure, which will increase the volume of contrast medium used with its inherent risk of contrast-induced nephropathy;18 the risk of jeopardising viable myocardium during revascularisation of a non-IRA; and the higher risk of stent thrombosis by operating in the highly thrombogenic peri-infarction milieu. More extensive acute revascularisation in patients with STEMI may be safer in the current era due to advances in stent technology and antiplatelet therapy, mainly in higher risk subgroups.19 This might reduce the duration of hospitalisation, resource utilisation and costs.
Available studies have excluded subjects with concurrent chronic total occlusion (CTO) lesions. This condition is a further independent predictor of both early and late survival.20 It is usually found in 10–15 % of patients with STEMI. The Percutaneous Intervention for Concurrent Chronic Total Occlusions in Patients With STEMI (EXPLORE) trial recently showed that additional CTO–PCI within 1 week after primary PCI for STEMI is feasible and safe, but does not infer a benefit in terms of left ventricular function or volumes.21
The main points of criticism of published studies are that no ischaemia testing nor guideline-based treatments (staged PCI) were performed in the control group, leaving potentially critical lesions untreated.12–14 This criticism can be partially overcome thanks to the MULTivessel Immediate Versus STAged RevaScularization in Acute Myocardial Infarction (MULTISTARS AMI) trial (NCT03135275), which is currently randomising individuals to immediate or staged CR. This study intends to include approximately 1,200 patients. It has a primary composite endpoint of all-cause death, non-fatal MI and unplanned ischaemia-driven coronary revascularisation. The on-going Complete vs Culprit-only Revascularization to Treat Multivessel Disease After Primary PCI for STEMI (COMPLETE) trial (NCT01740479) is randomising patients with STEMI to CR strategy with staged PCI of all suitable non-culprit lesions or culprit lesion-only revascularisation. The estimated enrolment is 3,900 individuals and the primary endpoint is the composite of cardiovascular death or new MI. The study should be completed in December 2018.
Although the fractional flow reserve (FFR) is infrequently evaluated in non-IRA in the setting of primary PCI, it may be helpful to assess the haemodynamic significance of potential target lesions. In this context, the Ffr-gUidance for compLete Non-cuLprit REVASCularization (FULL REVASC) study (NCT02862119) is currently randomising STEMI patients with MVCAD to a conservative strategy of IRA-only primary PCI or to FFR-guided ad hoc or staged revascularisation. This study will enrol an estimated 4,052 patients. The primary outcome is the combined endpoint of all-cause mortality and MI. It is anticipated that all of the data will be collected by October 2019.
In summary, based on current evidence, patients with STEMI and MVCAD should receive CR if admitted with cardiogenic shock or with persistent ischaemia after treatment of the culprit lesion. In haemodynamically-stable patients, multivessel PCI is a valuable option, either at the time of primary PCI or as a planned staged procedure during the same hospitalisation.
Complete vs Incomplete Revascularisation in Non-ST-Elevation Acute Coronary Syndromes
Non-ST-elevation acute coronary syndrome (NSTE-ACS) is the most frequent phenotype of acute coronary syndromes. Patients presenting with NSTE-ACS are a very heterogeneous group with a highly variable prognosis. In patients with NSTE-ACS, the optimal timing and treatment strategy in the presence of MVCAD is still unclear.1,22,23
In NSTE-ACS, the identification of the culprit lesion can be more problematic than in STEMI, as ECG is a poor predictor of IRA and ST-depression usually does not precisely localise in the myocardial territory with evolving ischaemia. Thus, the identification of the culprit lesion is usually achieved by a combination of factors including angiographic characteristics and information from an imaging technique.
MVCAD is present in approximately 50 % of patients with NSTE-ACS. In such cases, the revascularisation strategy is more complex and the choice has to be made between multivessel PCI, culprit-lesion-only PCI occasionally followed by a staged PCI, CABG or a hybrid revascularisation.1 Immediate CR during the index procedure can be more safely performed in MVCAD than with STEMI. Multivessel stenting is potentially associated with greater contrast load and periprocedural myocardial infarction,24 as well as with a higher risk of both later restenosis and stent thrombosis.25 Multivessel PCI, in contrast, has been repeatedly associated with lower death and MI at mid-term follow up.26–28 A functional assessment of lesion severity and myocardial viability is likely to be crucial to limit intervention to coronary segments expected to provide myocardial benefit from revascularisation, thus maximising the benefit and reducing risks. The Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME) trial has shown that FFR “re-categorises” patients otherwise classified as MVCAD by angiography, and that the treatment of lesions with a FFR <0.80 reduces long-term adverse events in comparison to the allegedly unnecessary deployment of stents in all lesions judged as severe only on the basis of angiography.29 As for the assessment of an “acceptable” angiography-based IR, Genereaux et al. proposed the residual SYNTAX (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) score, documenting a significant increased risk of 1-year death or MI for a value >8.30
In order to define the optimal timing of CR, the Single-Staged Compared With Multi-Staged PCI in Multivessel NSTEMI Patients (SMILE) trial randomised patients to immediate CR or to culprit-only revascularisation, followed by revascularisation of the remaining lesions during the index hospitalisation. Lower rates of major adverse cardiovascular and cerebrovascular events in the ad hoc group were documented at a 1-year follow up.31 However, the difference was essentially driven by the higher rate of target vessel revascularisation in the multistage group.
In some cases hybrid coronary revascularisation, combining minimally-invasive CABG to the left anterior descending (LAD) coronary artery and PCI of non-LAD arteries might offer potential advantages beyond CABG or PCI alone.32 Guidelines are inconclusive about the most appropriate timing of treatment in patients presenting with NSTE-ACS and MVCAD at present.22,23
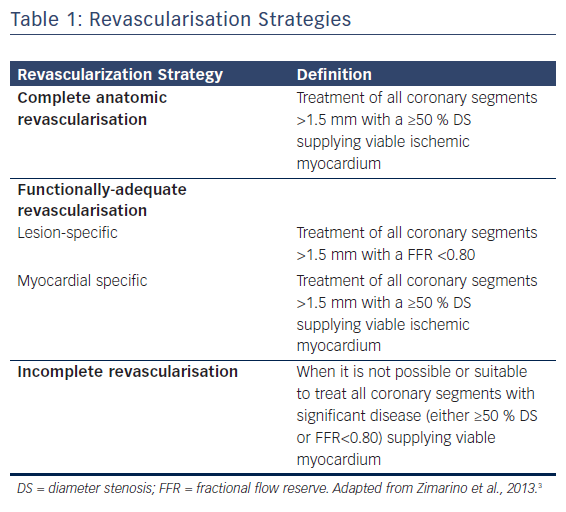
Concerning treatment strategy, in current guidelines CABG is recommended over PCI in patients with a baseline SYNTAX score >22, with left main or three-vessel coronary artery disease involving the proximal LAD. Ad hoc multivessel PCI, although extensively performed in low-risk patients (see Figure 1), is associated with increased risk of periprocedural damage and contrast-induced nephropathy. In patients with a SYNTAX score ≤22, a staged PCI aimed at treating all significant coronary segments supplying viable myocardium after an initial PCI directed only to the culprit lesion might be the strategy of choice as it reduces the procedural risk, dilutes the amount of contrast medium over time and allows the functional evaluation of “presumed non-culprit” lesion-related myocardial territories, as well as reducing patients’ symptoms.1,22,23
Complete vs Incomplete Revascularisation in Stable Coronary Artery Disease
Stable coronary artery disease (SCAD) is generally characterised by episodes of reversible myocardial demand/supply mismatch related to ischaemia. Episodes are usually inducible by exercise, emotion or other stress but may also occur spontaneously. SCAD also includes the stabilised, often asymptomatic, phases following an acute coronary syndrome.1,33,34
Guideline-directed optimal medical therapy (OMT) is recommended for all patients with SCAD because of the reduced risk of death and MI and the improvement of symptoms; however, 50 % of medically-treated patients have persistent symptoms within 1 year.34 The principal goal of revascularisation here is the relief of ischaemia to improve quality of life and exercise capacity, to reduce the amount of antianginal drugs, and ultimately to improve prognosis over and above the beneficial effects of medical treatment. Most recent international guidelines recommend myocardial revascularisation for patients with SCAD in the presence of flow-limiting lesions and limiting symptoms if individuals are unresponsive to medical treatment. There is substantial evidence supporting the association between the extent of myocardial ischaemia and the risk of cardiovascular events, as well as the direct relationship between the burden of ischaemia and the severity of prognosis.1,33,34
The most valuable source of data on an adequately-sized population of patients who have undergone either CABG or PCI is the randomised SYNTAX trial.35 This trial compared drug-eluting stent–PCI with CABG in patients with stable MVCAD.7 Patients were treated with the intention of achieving anatomic CR of all vessels ≥1.5 mm in diameter with stenosis ≥50 %. No functional evaluation was available. Individuals with less extensive MVCAD, as documented by a low (<22) SYNTAX score, had similar 1- and 3-year major adverse cardiac or cerebrovascular event rates after PCI and CABG. A higher 3-year risk of major adverse cardiac or cerebrovascular events has been documented after PCI compared to CABG for patients with intermediate (23–32) and high (>32) scores.36
Robust data now recommend functional evaluation of reversible myocardial ischaemia prior to elective revascularisation. It is now well documented that revascularisation of lesions that are not producing significant ischaemia confers a cost in terms of adverse outcomes.37 Here, OMT is a better alternative. Conversely, patients with moderate-to-severe symptoms and/or extensive ischaemia should be strongly considered for revascularisation therapy. Despite this recommendation, the majority of patients with SCAD undergoing elective PCI have no preceding documentation of myocardial ischaemia.38–40
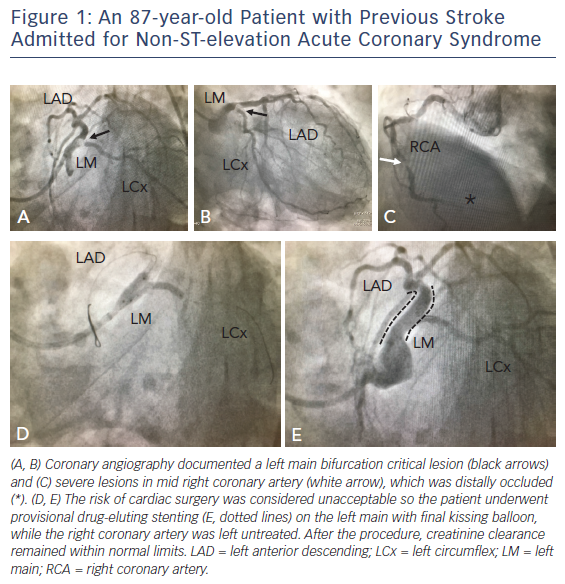
In this setting, accurate non-invasive (multidetector CT, stress echocardiography, perfusion scintigraphy, stress-MRI and/or PET-CT) or invasive (FFR, intravascular ultrasound, optical coherence tomography scan) identification of the lesions deemed responsible for ischaemia might improve outcomes in patients undergoing revascularisation. The availability and extensive validation of the FFR now offer a surrogate for ischemia and thereby represent an alternative to non-invasive stress imaging (see Figure 2).
In the FAME study, patients who had been listed for multivessel PCI were randomised to angiographically- or FFR-guided stenting (using a FFR cut-off value of 0.80).41 The FFR-guided strategy was found to be both cost-saving and cost-effective.42 The strategy was also associated with a significant reduction in the occurrence of the composite primary clinical endpoint of death, nonfatal MI and repeat revascularisation at 1 year (p=0.02), as well as a significant reduction in mortality plus MI at 2 years (p=0.02).43
A functional evaluation of lesion severity may now be obtained by non-invasive coronary imaging. The Determination of Fractional Flow Reserve by Anatomic Computed Tomographic AngiOgraphy (DeFACTO) trial documented that FFR can be non-invasively obtained with CT angiography and, together with CT itself, is associated with improved diagnostic accuracy and discrimination compared with CT alone for the diagnosis of haemodynamically-significant coronary artery disease.44 Finally, cardiac magnetic resonance is increasingly being utilised in ischaemic heart disease for both the detection of lesions producing ischaemia45 and the identification of myocardial viability.46
The importance of functional evaluation has been well demonstrated by the results of the FAME 2 trial.47 About 900 SCAD patients with functionally-significant stenosis (FFR ≤0.80) were randomly assigned to either OMT or OMT plus FFR-guided PCI, with predominant use of drug-eluting stents (>97 %). The study was stopped prematurely by the Data Safety Monitoring Board due to the significantly lower incidence of a composite of death, MI and urgent revascularisation favouring FFR-guided PCI plus OMT (4.3 %) as compared with OMT alone (12.7 %, p<0.001), although the result was driven by the “soft” component of urgent revascularisation. The on-going International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial (NCT01471522) should finally answer the fundamental question of whether myocardial revascularisation in addition to OMT in patients with moderate to severe ischaemia is associated with survival benefit compared with OMT alone.
Another point of criticism in achieving CR is the presence of CTO, which is frequently documented in patients with MVCAD (between 18 % and 52 % of cases).1,48 There is little consensus as to whether such lesions should be routinely treated by PCI.49,50 Potential benefits of successful PCI may include symptom relief, resolution of ischaemia and functional improvement. CTO–PCI has lower success rates than PCI of non-CTO lesions, with potentially serious complications; however, in high-volume centres with specific expertise, contemporary success rates of 80–90 % have been reported.51,52 In the setting of SCAD there is a clear lack of evidence, with randomised data derived from two unpublished trials. The optimal medical therapy with or without stenting for chronic coronary occlusion (Drug-Eluting Stent Implantation Versus Optimal Medical Treatment in Patients with Chronic Total Occlusion [DECISION-CTO], NCT01078051), although with some relevant limitations and despite a high technical success rate, failed to demonstrate a benefit with CTO–PCI on top of OMT. Recently released data from the Randomized Multicentre Trial to Evaluate the Utilization of Revascularization or Optimal Medical Therapy for the Treatment of Chronic Total Coronary Occlusion (EuroCTO, NCT01760083) showed that CTO–PCI improved quality of life, as assessed by the standardised Seattle Angina Questionnaire, in patients undergoing CTO–PCI. This benefit was coupled with a low rate of periprocedural complications. Further studies are necessary to assess the impact of PCI–CTO on symptom improvement and prognosis, especially in selected populations such as patients with high ischaemic burden. Currently, the use of CTO–PCI to improve quality of life should be restricted to selected patients in high-volume centres with specific expertise.
Current guidelines recommend CABG over PCI in patients with three-vessel disease, non-isolated left main disease or with involvement of the proximal LAD artery plus one other major coronary artery. CABG has to be considered in patients when in-stent restenosis recurrence is located on the LAD, where several devices have evenly failed.25 The benefit obtained with CABG seems to be larger with a systematic utilisation of the left internal mammary artery (IMA), an atherosclerosis-free conduit with patency rates >90 % at 10 years.53 Nevertheless, the Arterial Revascularization Trial (ART) recently failed to document any 5-year benefit for patients with MVCAD who received bilateral IMA as compared with single left IMA utilisation.54
The choice between revascularisation techniques should be based on several factors, including anatomical and clinical features. It requires a multidisciplinary approach to decision making by the heart team.
Conclusions
The extent of myocardial revascularisation is a major determinant of survival among MVCAD patients. Based on available evidence, revascularisation with either CABG or PCI has similar benefits in terms of survival in patients with MVCAD, with a prognostic advantage for CABG in the presence of more extensive disease and in patients with diabetes.
Both PCI and CABG should always aim at functional CR. Patients with diabetes and extensive coronary disease have a reduced risk of death and MI when they have undergone CABG, which attains CR more frequently than PCI. PCI is better suited in patients presenting with acute coronary syndrome and a suitable anatomy, where CR can be achieved through staged procedures that allow risk containment and the evaluation of both myocardial viability and lesion relevance.
The adequacy of myocardial revascularisation should be the priority when choosing between PCI and CABG in patients with MVCAD. A concerted multidisciplinary decision-making process should guide physicians, taking into account anatomy, left ventricular function, the extent of inducible ischaemia, myocardial viability, comorbidities and patients’ informed choices.