Acute myocardial infarction (AMI) is still a major public health problem worldwide, causing high rates of morbidity and mortality. In the United States, nearly one million patients suffer from AMI each year.1 In the UK, around 80,000 people died from coronary heart disease (CHD) in 2010.2
The current approach to the treatment of myocardial infarction involves early revascularisation with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), followed by the medical management of atherosclerotic risk factors, late ventricular remodelling and cardiac arrhythmias.
Improvements in the treatment of AMI, especially use of reperfusion therapy, have led to larger numbers of survivors. In patients who would have survived despite reperfusion therapy, use of this treatment should lead to greater myocardial salvage and a reduced extent of ventricular injury in many. However, others who might not have survived previously may now do so, but with substantial left ventricular damage.3,4 The net consequence of these two opposing effects on the early and later risk of developing heart failure after AMI is uncertain.
Several clinical trials and registries, despite methodological differences, tend to agree that heart failure is a common occurrence after AMI, and there has been concern that an increasing pool of survivors of AMI might fuel an ‘epidemic’ of heart failure.5,6
Patients with chronic heart failure (CHF) have a mortality of 20 % within the first year after diagnosis.2 CHF accounts for roughly 70,000 deaths in the UK each year, corresponding to an average of 190 deaths per day.2
Despite recent advances in medical and device therapy and improvements in care over the past 20 years, the outlook for patients with heart failure remains poor, and survival rates are worse than those for bowel, breast or prostate cancer.7–9 Therefore, any new treatment modality that benefits heart failure patients has the potential to result in a dramatic improvement in health outcomes and substantial cost savings for the community.
Ventricular remodelling after AMI involves replacing a significant amount of cardiomyocyte cell mass with fibrotic tissue, which results in contractile dysfunction. This degenerative process is not always irreversible; depending on the extent of damage and age of the subject, some spontaneous regeneration of the cardiac muscle may occur, which might be of key importance in the next generation of treatment modalities for such a severe and frequently deadly condition. The identity of the stem cells involved in cardiac repair is, however, still uncertain. Several novel treatment strategies are emerging, aiming at each stage of the pathological remodelling process, including stem cell treatments, paracrine signalling, microRNA-modification of key signalling events and tissue engineering. Cardiomyoplasty and stem cell therapy are generating great expectation to treat different types of cardiac diseases, including AMI, refractory angina and CHF. Effective medical treatments of these conditions will produce crucial improvement in overall health outcomes and substantial cost savings for the National Health Service (NHS).
Pathophysiology of Myocardial Infarction
Myocardial ischaemia may result from either a rise in metabolic demand or a reduction of oxygen and nutrient supply to the myocardium. Myocardial infarct occurs if the demand/supply mismatch is enough to trigger cellular necrotic and apoptotic mechanisms within cardiomyocytes. Several conditions are associated with an increased myocardial metabolic demand, such as severe hypertension, severe aortic stenosis, other valvular pathologies and low cardiac output syndromes. Not only do these conditions increase the metabolic demand, but they also have the capability to reduce the coronary perfusion by lowering the mean aortic pressure. Infarction can also be caused by other conditions that are characterised by thromboembolic or atherosclerotic stenosis/occlusion of coronary arteries, leading to ischaemia primarily by decreasing the delivery of oxygen and nutrients to the myocardium.10
Myocardial Repair after Myocardial Infarction
There are several cellular changes that occur in the myocardium following myocardial infarction. During the first 6–12 hours, a process of coagulative necrosis occurs, and the fibres at the periphery of the infarct become elongated and narrowed with signs of vacuolar degeneration. Concomitantly, oedema and neutrophils are observed in the intercellular spaces. This process lasts for 3 to 4 days. Following this stage, the necrotic myocytes are removed by macrophages, which may be actively phagocytic for 7 to 10 days. Finally, granulation tissue with loose collagen fibres and copious capillaries commence the healing and repair processes, in which the necrotic cardiac muscle cells are replaced by a collagen scar.10
Cardiac Regeneration and Cell Therapy
The heart, which had been considered as a terminally developed organ with no potential for regeneration in post-natal life, has recently been recognised to possess some intrinsic reparability. Currently, there are two complementary theories about the process of intrinsic repair in the heart after an ischaemic injury: (1) cardiomyocytes re-enter the cell cycle and start the process of proliferation, regeneration and repair of the necrotic tissue;11,12 and (2) certain endogenous cardiac stem cells undergo growth and differentiation, regulated either by secreted inflammatory factors or autocrine regulation.13,14 Both mechanisms may be involved in the process of heart regeneration.15 Currently, the research focus is on how to translate the preclinical cell-based results into effective clinical treatment. In order to repair the human heart, it is crucial to identify the appropriate cell type and optimal route to deliver it. The selected cells should be able to differentiate into mature cardiomyocytes and achieve electrical integration with mechanical coupling. They should also have the capability to repair the heart via paracrine effects. Importantly, delivery of such cells should be done with careful consideration of the risks and benefits to the patient. Possible delivery methods include intravenous, intracoronary or intramyocardial.16 In selecting appropriate cells, one needs to know each cell’s individual potential: its regenerative activity (ESC, iPSC, and endogenous cardiac stem cells), paracrine effects (MSC) and angiogenesis activity (endothelial precursors).
Endogenous Cardiac Progenitors There are three different embryonic cardiac cell precursors: the cardiac mesoderm; the neural crest cells; and the pro-epicardial territory. Each of the original precursors will turn into different cardiac structures, as follows. (1) Cardiac mesoderm becomes endocardial cells, atrial myocytes and ventricular myocytes. (2) Cardiac neural crest becomes aorta smooth muscle cells and autonomic nervous system. (3) Pro-epicardium becomes smooth muscle of coronary arteries, fibroblasts, endothelium of coronary arteries.17–19
Recently, multipotent stem cells were identified in each one of the layers of human blood vessels. Myogenic endothelial cells (MECs) are located in the intima of blood vessels, whereas pericytes and adventitial cells (ACs) are located in the media and adventitia, respectively. MECs and pericytes have the capability to regenerate myofibres in dysfunctional skeletal muscles and to improve cardiac contractility following AMI.19
Recently, cardiogenic progenitor cells (CPCs) were detected in the adult heart. It is still not completely clear whether CPCs originate from the bone marrow, or there are populations of embryonic cells localised in the right atrium and right ventricle. Also, there is still ongoing research to determine the participation of these cells in the physiological turnover of cardiac myocytes and vascular endothelial cells in the absence of myocardial injury.20 CPCs represent 1 % of the total cell population in the heart and are divided into three groups so far identified (c-Kit+, Sca-1+ and ISL-1+ cells) according to the expression of membrane markers.20 c-Kit+ cardiogenic stem cells express pluripotency, clonogenicity and self-renewal capabilities, and differentiate into myogenic, vascular endothelial and smooth muscle lines in vitro. These cells can regenerate the ischaemic myocardium in animal models.21,22 The group of CPCs expressing Sca-1+ interact with a homogeneous cell population in foetal and adult human hearts and show self-renewal properties together with active participation in cell signalling and cell adhesion.23
It is possible to differentiate Sca-1+ CPCs into cardiomyocytes by using 5-azacytidine. 5-Azacytidine is similar to cytidine, a nucleoside found in either DNA or RNA. The mechanism of action of this drug is through inhibition of the enzyme methyltransferase. 5-Azacytidine is incorporated into the structure of DNA and RNA instead of cytidine, inhibiting the synthesis of proteins within the cells.24 Additionally, the activation of extracellular signal-related kinases (ERK) by 5-azacytidine seems to trigger the differentiation of human MSCs into cardiomyocytes in vitro.25 In vitro differentiation to cardiomyocytes appears to involve the receptor for bone morphogenic proteins like BMPR1A.26 Differentiated murine Sca-1+ cells can be detected as mature cardiomyocytes after intravenous transfusion following myocardial ischaemia and necrosis in rats.26
A group of stem cells is found in the hearts of newborn mice, rats and humans. Neonatal mouse hearts have cells that express the transcription factor ISL-1 together with two more factors: Nkx2.5 and GATA4, which are crucial transcription factors that participate actively in the initial stages of cardiogenesis, but don’t express either c-Kit or Sca- 1.26,27 These cells can differentiate into cardiomyocyte phenotypes with intact calcium cycling. They produce action potentials when cultured together with neonatal myocytes.27,28 These findings allow the study of the molecular pathways linked to the differentiation of ISL-1+ cells into the different lineages in either postnatal or embryonic hearts. The limited capacity of human cardiomyocytes to regenerate in vivo is responsible for the development of heart failure after infarction. Understanding the molecular mechanisms involved in the differentiation of the embryonic heart is of crucial importance in the design of effective regenerative stem cell therapies to treat patients with cardiac injury.
Selection of Cell Types
There are two important mechanisms by which stem cells may work. (1) Paracrine effect of the cells: SKMs, BMMNCs and MSCs produce several cytokines and growth factors that increase angiogenesis, reduce apoptosis, decrease fibrosis and induce cardiac regeneration. Ischaemic patients can especially benefit from the paracrine effect, which enhances perfusion.29–31 (2) Trans-differentiation of the stem cells’ phenotypes into cardiomyocytes and replacement of injured cells, increasing the contractility of the injured tissue.
Bone marrow MSCs, adipose-tissue-derived stromal cells and pericytes are known to produce cardio-protective cytokines that could be enhanced by genetic engineering.30–32 These cells also have immunosuppressive properties, which allows their usage as potential allogenic drugs.33 Additionally, the cell factors can induce regeneration from myocardial niches of tissue-resident stem cells. The paracrine effect alone would not be enough to relieve severe heart failure with extended scars as it would require cardiac regeneration to complete the healing process. The cells should be able to contract and coordinate each other through Connexin-43, a protein involved in the myofibrillar coupling structure, thus avoiding lethal arrhythmias.34
Cardiac-committed stem cells could be extracted from endomyocardial biopsies or during CABG, expanded in vitro and reinjected. Current clinical human trials, such as Stem Cell Infusion in Patients with Ischaemic Cardiomyopathy (SCIPIO: cells harvested from right atrial appendage during CABG, which uses c-Kit + CSCs) and Cardiospherederived Autologous SCs to Reverse Ventricular Dysfuntion (CADUCEUS: endomyocardial biopsy, which uses CDCs), have been showing promising results.35,36 In these trials, the cells expanded in vitro are injected into the coronary arteries in the catheterisation laboratory. In contrast, the Autologous Human Cardiac-derived Stem Cell to Treat Ischaemic Cardiomyopathy (ALCADIA) trial involves the delivery of the cells into the myocardium during CABG. Cardiac-derived stem cells are extracted from endomyocardial biopsies, expanded and then delivered to the heart during CABG surgery by intramyocardial injections then a biodegradable gelatin hydrogel sheet containing fibroblast growth factor is implanted on the epicardium.37 The ongoing problem is to clarify the characterisation of the cell phenotypes, as current phenotypic differences could correspond to the same cell in a different stage of development.38
The CADUCEUS trial uses a mixed population of stem cells denominated by cardiospheres of which mesenchymal stem cells are a big proportion.39,40 The SCIPIO trial works with c-Kit cells; whereas ALCADIA uses a mixed population extracted from endomyocardial biopsies and cultured for a month.
Within the pool of pluripotent stem cells, human ESCs could be committed toward cardiac lineage in vitro. These cells were obtained from disposed embryos in the context of assisted fertilisation. Results show good engraftment of differentiated cardiomyocytes, although the risk for teratomas and immune rejection needs further investigation.41–43
Another source of potential cells could be the pool of iPSCs that are selected from the patient’s somatic cells and reprogrammed to embryonic pluripotent status. Because of their oncogenic potential, they still need larger animal trials before they can be introduced to the market.44 MSCs and fibroblasts could be manipulated in vitro towards enhanced cardiopoiesis, thus increasing the intrinsic therapeutic benefit of the treated cells.45–47
Perivascular/Mesenchymal Stem Cells
The pericyte is the second most common cardiac cell type and its participation in cardiac pathophysiology and regenerative medicine is crucial.48 Pericytes are perivascular cells with contractile capability similar to those of the smooth muscle cells that wrap around blood vessels. These cells carry out several functions, including active participation in the development of vessels and their structural maintenance. Additionally, they can communicate with surrounding cells during the angiogenic process, either by direct contact or paracrine signalling.49 New insights into the use of pericyte transfusion as a potential new treatment for AMI showed that there was a significant improvement in the infarcted heart in a mouse model. The effect was achieved through lowering the threshold and the activation of an angiogenic program in the recipient model.50
The identification of pericytes in tissue is a complex process because there is no single reliable marker. Currently, several markers, such as NG2 (neuron-glial antigen 2), α-SMA (alpha smooth muscle actin) and PDGFR-β (beta-type platelet-derived growth factor receptor), are used, each staining a subset of pericytes. Additionally, CD146 stains pericytes and a subset of endothelial cells; CD34 stains endothelial and progenitor cells; and CD31 and CD144 stain mature endothelial cells. Histologically, pericytes are identified as cells positive for CD146 but negative for endothelial markers such as CD34, CD31 or CD144. The NG2 marker is a chondroitin sulphate proteoglycan that can be found on the surface of pericytes and a small subset of glial and endothelial cells and is expressed by SMA-negative pericytes, either in micro-vessels or in the intimal layer of large vessels.51 α-SMA is present in vascular smooth muscle cells and in pericytes. This marker was identified in the microfilament bundles responsible for pericyte contractile functions.52 PDGFR-β is a useful abundant pericyte marker.53 Pericytes from mice that have abnormal PDGFR-β receptors exhibit micro-vascular abnormalities leading to lethal micro-haemorrhages and oedema.54 CD146, also known as Mel-CAM, MUC18, A32 antigen and S-Endo-1, is a specific membrane glycoprotein that can function as a Ca2+ independent cell adhesion molecule with participation in heterophilic activity between cells. CD146 is part of the immunoglobulin gene superfamily.55 CD34 is a trans-membrane protein expressed in either haematopoietic progenitor cells or vascular endothelial cells. In addition, CD34 takes an active part in the regulation of cell migration and differentiation.56
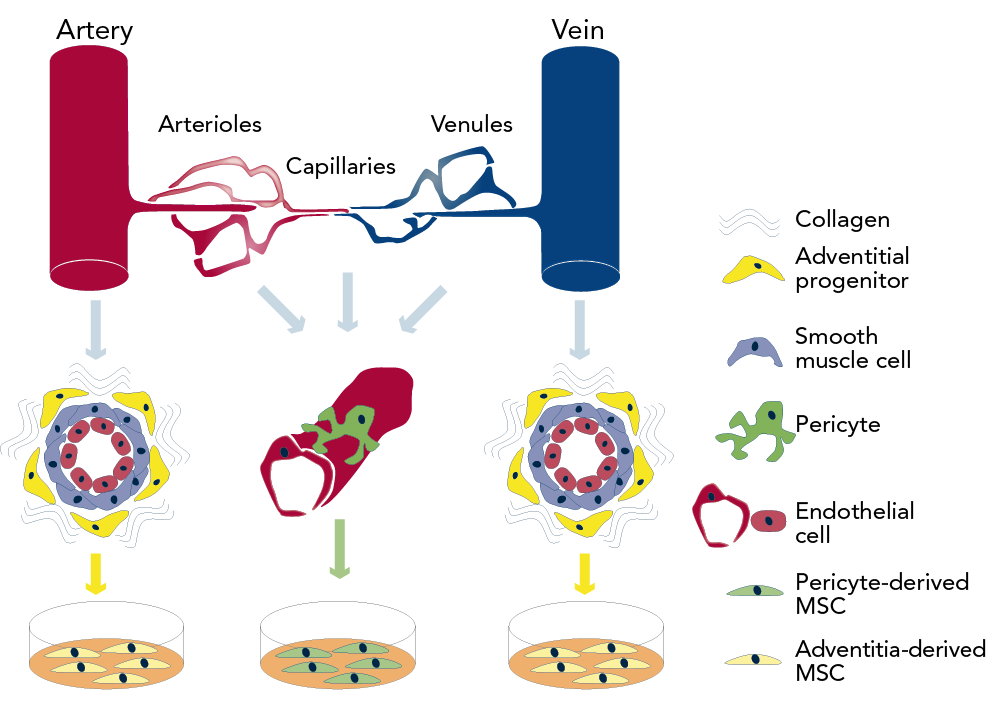
Pericytes have been identified in several human organs, including skeletal muscle, pancreas, adipose tissue and placenta, using markers such as CD146, NG2, and PDGFR-β and absence of haematopoietic, endothelial and myogenic cell markers.57 Recent research demonstrated that human pericytes that are located around capillaries and micro-vessels can produce MSCs while in culture. Additionally, during the process of vascular regeneration and under the effect of growth factors, adventitial cells can undergo a phenotypic trans-differentiation into pericyte-like cells (see Figure 1).58 Furthermore, there is clear evidence that pericytes contribute to cardiac repair by down-regulating immune cells via interaction with immunomodulatory cytokines and growth factors, following pericyte injection into ischaemic tissues.59
Clinical Applications
Current treatment protocols for AMI focus on reducing myocardial necrosis and irreversible damage by improving perfusion to the ischaemic area via medical or mechanical treatment such as CABG or PCI.60–62
The new potential cell-based treatments to deal with AMI derive from animal research in which mononuclear cells from bone marrow or peripheral blood were used in cardiac repair.63–68 Ongoing research in cardiac developmental and stem cell biology, as well as recent results from clinical trials SCIPIO and CADUCEUS using resident cardiac stem cells, have improved our understanding of in situ heart stem progenitor cells.69,70 The first non-randomised trials in humans showed that there was an improvement in cardiac function after the infusion of bone marrow stem cells and progenitor stem cells into the myocardium affected by the infarction.71–75 The stem cell types involved in the repair of cardiac tissue were first characterised by Stamm’s group in 2003, when the infusion of CD133+ progenitor cells extracted from haematopoietic tissues were applied into the ischaemic cardiac myocardium. The result of this treatment was an improvement in the general revascularisation process.76 The first randomised multicentre trial in 2009 studied patients with severe left ventricular dysfunction as a consequence of AMI. The patients, who were infused with selected CD34+ and CXCR4+ cells and non-selected mononuclear cells into the lumen of their coronary tree, saw significant improvement in their left ventricular ejection fractionafter 6 months.77 The mechanism of action of such treatment seems to be either an increase in the angiogenesis activity and/or trans-differentiation of the cells into myocytes.78 The paracrine secretion of cytokines and other factors also increases vascular growth, favours cardiac repair and reduces local fibrosis.79 Latest evidence from trials shows that adult bone marrow stem cell treatment significantly improves cardiac function in post MI patients and there is no evidence of any increase in morbidity or mortality in this treated group of patients.80 Research into more effective stem cell treatments allowed the isolation of neonatal and ischaemic myocardial cells expressing the c-Kit, MRD-1, ISL-1 or Sca-1 stem cell markers but no haematopoietic cell markers.26,81 The number of these cells increased after an AMI, suggesting an active role of these cells in cardiac repair.82
Application in Acute Myocardial Infarction with Concurrent CABG
Intra-myocardial injection of BMMNCs during CABG is shown to have improved outcomes compared with those of CABG alone.83 The aim of treating patients with stem cells after or during CABG following an episode of acute MI is to reduce later remodelling, which is known to have a negative impact on long-term outcomes.83 Such treatment is carried out by the interventional cardiologist and consists of delivering BMMNCs into the new coronary bypass graft. Unfortunately, there is still a need for more randomised trials to assess the potential benefits currently observed.83–85 Patients with poor left ventricular function undergoing CABG seem to be better at 6 months post-operative if trans-epicardial injection of CD133+ cells was performed intraoperatively.84,85 These observations likely result from the angiogenic potential of cells rather than cardiomyocyte regeneration, since the CD133+ marker is expressed in the membrane of the endothelial cells. The PRECISE (Percutaneous Robotically-enhanced Coronary Intervention) trials use adipose-tissue-derived cells collected with lipo-aspiration from patients at the time of surgery. These cells are subsequently reinfused into the endocardium of the left ventricle. The final results of this technique are still pending.86
Application in Refractory Angina
A second treatment indication under investigation is for refractory angina (angina caused by coronary insufficiency in the presence of coronary artery disease that cannot be controlled by a combination of medical therapy, angioplasty and coronary bypass surgery), which would involve stem cell treatment alone or complemented with surgery. The aim in this subset of patients would be to use the different cell types as carriers of multiple cytokines and growth factors in order to induce angiogenesis in the affected territory and thus relieve ischaemic symptoms.87,88 Patients with refractory angina are currently under investigation in randomised trials to assess the apparent efficacy of catheter-based endoventricular injection of CD34+ cell progenitors following treatment with granulocyte colony stimulating factor for 5 days in order to induce autologous cell mobilisation.89 Another randomised trial in the population of patients with refractory angina used trans-cathether endomyocardial injections of bone marrow unfractionated derived cells (MNC), which seem to have some efficacy in improving clinical parameters but more data is needed to find significant differences between the study arms.90
Application in Chronic Heart Failure
A third application under research is the treatment of chronic heart failure patients in whom the aim is to regenerate areas of non-contractile myocardial fibrosis to achieve physiological and functional contractility.88 Patients with chronic heart failure were included in the randomised, double-blinded placebo-controlled Myoblast Autologous Grafting trial. In addition to CABG, patients with severe left ventricular function underwent trans-epicardial injections of either autologous SKMs from a skeletal muscle biopsy or placebo injected in and around the scar.91
The preliminary results showed that there was no improvement in the ejection fraction at 6 months, but patients injected with 800 million cells presented a reduction in left ventricular volumes.91 The effects of such treatment on early post-operative rhythm abnormalities and left ventricular remodelling require further investigation. Intracoronary injection of bone-marrow-derived cells with and without CABG was tested in trials, but the results remain inconclusive.92,93 Regarding complications related to the type of cells, ventricular arrhythmia with myoblast implantation is the most worrisome.
Future Prospects
The future of cardiac repair may rely on understanding the intrinsic mechanisms that regulate endogenous mobilisation and or delivery of these cells. Additionally, further studies are needed to develop a deeper understanding of the properties of pericytes and their potential to migrate to different tissues away from their perivascular location and to play an active role in cardiac repair after ischaemia.49 This would involve a more modern interpretation of the pericyte’s role as a cell type involved in reducing the threshold for the activation of an angiogenic program in cardiac repair.50 It has been shown that exogenous administration of MSCs could stimulate cardiac precursors to proliferate and differentiate either by stimulation of the endogenous c-Kit+ CSCs or by improving cardiomyocyte cell cycling.94
Despite advancements in the field of cardiac regenerative biology, the perivascular cell compartment within the myocardium and its regenerative capability have not yet been studied in-depth. Pericytes and perivascular cells have a crucial role in physiological functions, as well as in the development of pathological conditions.95–98 Additionally, the participation of perivascular cells in post-injury tissue fibrosis has been shown in recent studies. The cardiac pericyte is the second most common cardiac cell type, and has started to attract attention in cardiac pathophysiology and regenerative medicine.99 There is ongoing research on the role of pericytes in the activation of endogenous cardiac progenitors during cardiac repair.
Recently, there has been increasing interest in the study of transcription factors and signalling pathways involved in cardiac regeneration. This has triggered the investigation of thymosin β4, which is a protein that can reactivate the cells’ embryonic developmental potential and stimulate epicardial cell trans-differentiation to vascular regeneration.100–103
Conclusions
Autologous cardiac cellular therapies appear to be safe and effective. The future of cardiac repair may rely on understanding the intrinsic mechanisms that regulate endogenous mobilisation and/or delivery of these cells. However, a considerable amount of work is to be performed before cardiomyoplasty (cell therapy of the heart) can be proposed as a routine treatment. The first question is which cells to use, as a variety of embryonic stem cells, reprogrammed adult stem cells, natural adult multi-lineage stem cells and lineage-committed stem cells are presently available. Arguably, the latter endogenous cardiomyogenic stem cells might be the best choice for cardiac repair. Ideally, these cells should be directly stimulated in situ, avoiding extraction, purification, culture and reinjection. It is therefore of uttermost importance to understand the identity and function of the cells that constitute the natural environment of cardiac progenitors and support their quiescence, self-renewal and activation. Additionally, further studies are needed to develop a deeper understanding about the properties of pericytes, as these cells have the potential to migrate to different tissues away from their perivascular location and play an active role in the activation of cardiac repair after ischaemia.49 This would involve a modern interpretation of the pericyte’s role as a cell type involved in reducing the threshold for the activation of an angiogenic program in cardiac repair.50