The US Public Health Service defines cardiac rehabilitation services as “comprehensive, long term programs involving medical evaluation, prescribed exercise, cardiac risk factor modification, education and counselling. These programs are designed to limit the physiological and psychological effect of cardiac illness, reduce the risk of sudden death or re-infarction, control cardiac symptoms, stabilise or reverse the atherosclerotic process and enhance the psychological and vocational status of the individual patient.”1
Exercise training is a core component of comprehensive rehabilitation programmes. It is currently recommended in combination with pharmacological therapy to patients with chronic heart failure (CHF) with reduced ejection fraction (EF) at a class 1 evidence level.2,3 The benefits of exercise-based cardiac rehabilitation for clinically relevant health outcomes (e.g., functional capacity, exercise tolerance and quality of life) have been widely recognised in CHF patients.4 There has also been a positive effect on heart failure (HF)-related hospitalisation and mortality.5–7 The optimum “dose” (volume and intensity) of exercise is still being questioned as well as the correct amount that should be prescribed to improve health outcomes.
Defining the optimal dose of exercise to maximise health outcomes is now considered a priority.8–10 Current guidelines for exercise prescription are commonly based on heart rate (HR): either a percentage of peak heart rate (HRmax) or heart rate reserve (HRR; the difference between resting HR and HRmax), as determined in a symptom-limited exercise test.11 Target intensity generally ranges between 70 and 80 % of HRmax, for at least 30 min on >5 days/ week. Exercise training guided by HRmax or HRR can be limited in CHF patients due to chronotropic incompetence and beta-blocker treatment. It is important to recognise that contributions documented for each patient group may not fully apply to each member, despite all patients being exposed to the same volume and intensity of physical activity adjusted for their own tolerance level. For example, a group of CHF patients exercising at 40–70 % HRR may be working at individually different relative intensities. For this reason, intensities based solely on a percentage of HRR or HRmax are likely to impose variable cardiovascular and metabolic demands in CHF patients.12,13
In addition to the dose, the best format of aerobic exercise training has not been defined. Training programmes in CHF patients have been based predominantly on continuous, moderate and vigorous aerobic exercise.11,13 However, a study by Wisloff et al. reported a greater benefit in terms of functional capacity with interval training as opposed to moderate continuous aerobic exercise training programmes in patients with CHF.14 The interval training included alternations of 3–4-minute periods of exercise at 90–95 % HRmax with exercise at moderate intensity (60–70 % HRmax). Since the study by Wisloff et al., interval training has gained popularity.
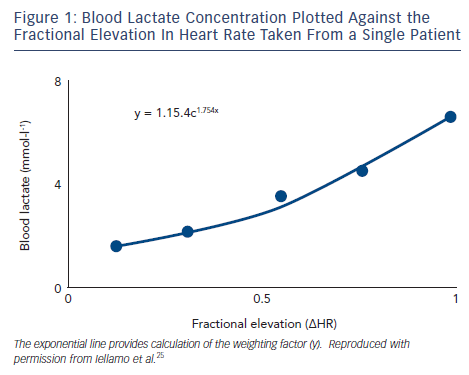
In the past few years, our group has systematically addressed the above issues by applying a training method that takes into account the dose of exercise (both the volume and intensity) on an individual basis. This method has been referred to as individual TRaining IMPulses (TRIMPi)15 and represents an individualisation of the TRIMP method originally proposed by Bannister et al.16 The original TRIMP considers the ∆HR (HRexercise-HRrest/HRmaximal-HRrest) as the main exercise variable. The duration of any specific training session is multiplied by the average ∆HR achieved during that session. The ∆HR is weighted by a multiplying factor (y) in a way that reflects the intensity of effort. This avoids providing a disproportionate conclusion to long-duration activity at low ∆HR levels compared with intense but short-duration activity. The y factor is based on the exponential rise of blood lactate levels and the fractional elevation of exercise above resting HR. It is computed using two constants in the equation that are considered equal for all subjects.15 The use of the same multiplying factor y can potentially overlook the individual physiological demands of each training session. We introduced an individual weighting factor (yi) that is calculated for each patient with the best fitting method using exponential models (see Figure 1). Thus, as exercise intensity increases, as indicated by the HR response, the weighting factor yi increases exponentially. As a result, during each training session a unique TRIMP can be calculated at any time from the area under the curve represented by the pseudo-integral of all ∆HR data points.15 We used the TRIMPi method to quantify the training load and its effect on the physiological systems in CHF patients with reduced EF in a series of investigations that are the focus of this review.
Cardiorespiratory and Metabolic Adaptations
To define the best dose and modality of exercise on health parameters, we investigated the effects of both aerobic continuous training (ACT) and aerobic interval training (AIT) on haemodynamic, cardiorespiratory and metabolic adaptations in patients with CHF under optimal therapy (including beta-blockers). We did this using a randomised design.17 The TRIMPi method was employed to provide equivalent doses of exercise in both ACT and AIT.
In this study, the increase in functional capacity and ventilatory efficiency to exercise training did not differ between continuous and interval training modes. Resting central haemodynamics were not significantly affected by either ACT and AIT, as indicated by the lack of changes in stroke volume, cardiac output, ejection fraction and left ventricular diastolic diameter in both patient groups. This finding is in line with most studies, indicating that the beneficial effects of exercise training on functional capacity and ventilatory efficiency in patients with CHF would be mainly due to peripheral mechanisms and adaptations,18–21 although not invariably.21,22 The effect of ACT and AIT on metabolic parameters also did not differ significantly. It thus appears that adaptations to exercise training do not differ between ACT and AIT modes, provided that the training stimulus is equated by an individually tailored dose of exercise.
It should be outlined that, until recently, the relationship between training and functional capacity has been investigated independently from exercise mode without regard for individual training dose. Indeed, aerobic training prescriptions based on VO2max (maximum oxygen uptake) or HRmax percentages may result in different physiological responses, even in patients with similar baseline aerobic capacity. These differences in exercise responses might be the consequence of differences in individual internal training load. Scharhag-Rosenberger et al. recently reported a broad inter-subject variability in lactate response in individuals with similar aerobic capacity while exercising at the same percentage of VO2max.23 Hence, prescription of endurance training should not be solely based upon percentages of VO2max or HRmax (or HRR) when a comparable metabolic load is intended.
In this context, there is a general consensus that exercise training should be individually tailored to each patient’s clinical and functional status.8–10 In addition, exercise training programmed at given percentages of pre-training VO2max or HRmax is based only on pre- intervention criteria that are not updated during the training process to account for the increments in aerobic fitness documented at the end of the training intervention, as it is with the TRIMPi method. The TRIMPi approach allows a close monitoring of the internal load experienced by patients with the progression of training and enables accurate weekly training load adjustments within both continuous and interval training modes.
AIT has gained popularity9,10,18 since the Wisloff et al. report of a greater improvement in aerobic capacity with AIT than ACT in a small group of patients with CHF.14 Differences in the method of quantification of the training stimulus and prescription might be the main cause of this discrepancy. In the study by Wisloff et al.,14 in which the intended difference in the training stimulus between AIT and ACT was only the intensity of exercise (i.e. maintenance of the same level of energy expenditure in the two groups), the training load was actually greater in the AIT than in the ACT group, as we demonstrated.17 Furthermore, the training programme was not updated during the training process.
Our finding of a similar increase in functional capacity with both ACT and AIT coincides with the multicentre Study on Aerobic INTerval EXercise training in CAD patients (SAINTEX-CAD) performed in patients with coronary artery disease with preserved left ventricular function. The patients were exercising with progressive optimisation of HR training zones during the programmed ACT.24 If the target HR zones were not changed, this could have resulted in low intensities of ACT than AIT and, perhaps, smaller improvements in VO2max after intervention.24 Thus, it appears that in patients with post-infarction CHF, functional capacity, ventilatory efficiency and metabolic adaptations to exercise training do not differ between continuous and interval training modes, provided that the training stimulus is matched.
Autonomic Nervous System Adaptations
In addition to reduced exercise tolerance and functional capacity, patients with CHF also exhibit an increased susceptibility to life- threatening arrhythmias and sudden death. Alterations in the autonomic control of the heart, characterised by a relative sympathetic predominance and a decreased vagal modulation, play a major role in the occurrence of arrhythmic events.4,25 Depressed heart rate variability (HRV) and baroreflex sensitivity (BRS) are two clinical indices of vagal control of the sinoatrial node that are linked to a greater risk of ventricular fibrillation,26,27 they also represent a dominant feature of the CHF syndrome, which parallels deterioration of CHF status28 and carries a negative prognosis.28–30
Exercise training has been reported to improve HRV and BRS in patients with CHF31–33 and may ameliorate the increasing cardiovascular risk linked with autonomic derangement of patients with CHF.27,33 However, the optimal dose of exercise required to achieve improvement in neural cardiovascular regulation has not yet been defined. Not one study addressed whether a dose–response relationship exists between training load and improvements in autonomic nervous system (ANS) parameters and their relationship with performance in patients with CHF.
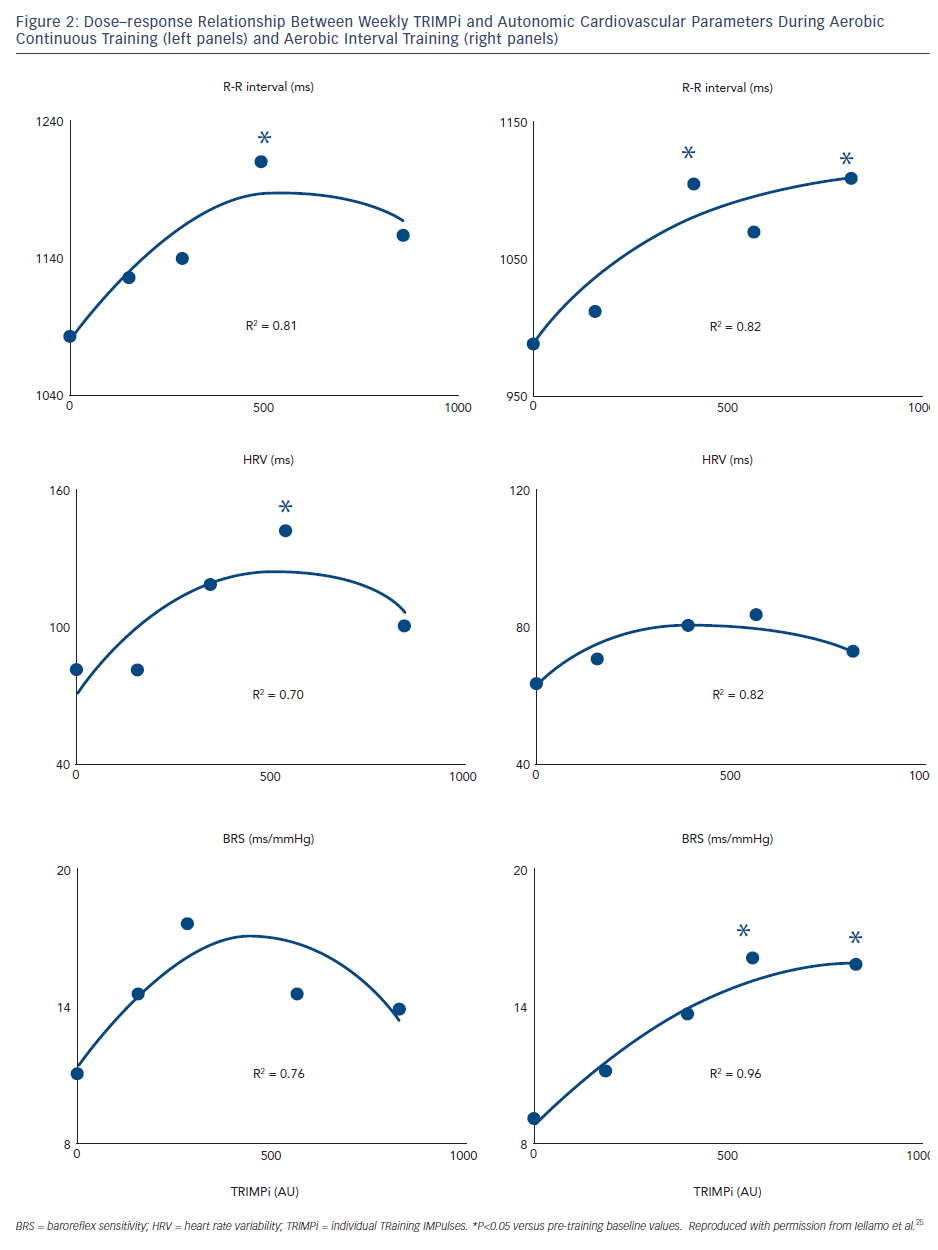
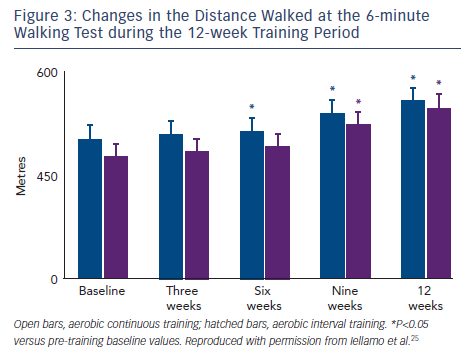
Our group used the TRIMPi method to address the issue of dose– response relationship of BRS and HRV to individually tailored exercise training in patients with CHF.24 We observed that BRS, HRV (standard deviation of mean R-R interval) and R-R interval as well, were significantly and very highly correlated with the dose of exercise, with a second-order regression model (r2 ranged from 0.75 to 0.96; P<0.001), resembling a bell-shaped curve in the ACT and an asymptotic-shaped curve in the AIT groups, respectively, without differences between exercise training modalities (see Figure 2). In the same study, a progressive increase in the distance walked in the 6-minute walking test (6MWT) was observed with the increase in TRIMPi, which was significant after nine weeks of training, without significant differences between ACT and AIT from the baseline throughout the study. A small, not significant, increase in the 6MWT was observed between the ninth and twelfth weeks of training with both protocols (see Figure 3).
These findings indicate that in patients with CHF under beta-blockers therapy, HRV and BRS adaptations to exercise training are dose related in a non-linear fashion on an individual basis and that higher doses of exercise training do not necessarily lead to greater improvement in HRV and BRS. Thus, a moderate dose of exercise, approximately 55–60 % of HRR for 40 to ~45 minutes four times a week, is sufficient to achieve substantial improvements in HRV and BRS. This dose maximises effects in patients with CHF; no further substantial improvements are made by employing more vigorous physical activity. This dose is sufficient to increase cardiac vagal activity, as reflected by HRV and BRS, and protect against life-threatening arrhythmias. More vigorous physical activity might pose a risk for arrhythmic events in high-risk populations like CHF patients. Previous studies in cardiac patients included only before and after training ANS assessments, which prevented the appreciation of the non-linear dose–response relationship between training load and ANS responses.
Interestingly, the above findings resemble those observed in marathon runners15 and otherwise healthy individuals of different ages and physical fitness levels who have undergone similar, although not individualised, training methods34,35 and experienced a decrease in HRV and BRS at the highest training loads. In line with these findings, Iellamo et al. reported a decrease in vagal cardiac modulation with a shift toward a sympathetic predominance with strenuous exercise in comparison with moderate training loads in world-class endurance athletes.36 From all the above studies, it appears that despite a prominent plasticity of cardiac autonomic regulation, there is a point on the dose–response curve above which an enhanced vagal modulation of HR ceases to occur with the increase in training load. Therefore, a higher exercise dose may not provide any added protective benefit by these mechanisms in patients with CHF.
There is a minor increase in functional capacity with increase in TRIMPi from moderate to higher values (see Figure 3). However, the potential benefit of increasing exercise performance by increasing training load from moderate to high doses should be weighed against the lack of improvement, even a decrease, in cardiac vagal modulation. This is coupled with the inherent, possible increase in the risk of adverse events in a high risk population, such as patients with CHF.
Subjective-based Exercise Training Prescription
It is well established that to be effective over time, exercise training should be a lifelong commitment. This would imply the need of a continuous adaptation of exercise prescription, in terms of volume and intensity, especially in ageing individuals. The exercise training methods described above are usually prescribed in clinical rehabilitation centres, where patients are admitted as in- or outpatients for a limited period of time. The issue thus exists on how prescribing long-term exercise training, taking into account the need of practising regular physical activity outside medically supervised settings and physiological ageing processes, with the attendant changes in individual physical capacity over time.
The session-rate of perceived exertion (RPE) method might potentially be used by cardiac patients for long-term, self-selected physical activity management. The session-RPE is a simple method to quantify internal training load proposed by Foster et al.37 By this method, internal training load is quantified by multiplying the perceived effort of the whole training session (using the Borg category ratio scale; the CR10 scale) by its duration.38 This product represents in a single number the magnitude of internal training load, in arbitrary units (AU), and has been used and validated in athletes of different sport disciplines.39–41 The perceived exertion method that uses RPE as a marker of training intensity within the TRIMP concept37 has a physiological basis as it has been shown to be related to both HR and blood lactate markers of exercise intensity during continuous and interval running.42
Borg’s CR10 is considered a global indicator of exercise intensity including psychological and physiological factors (oxygen uptake, HR, ventilation, beta endorphin, circulating glucose concentration and glycogen depletion).43 It can be considered an accurate indicator of global internal training load. Subjects are asked to provide a global rating of the effort for the entire training session, rather than the perceived effort of the most recent exercise intensity, using whatever cues they feel to be appropriate. To facilitate a global effort assessment, subjects report their global RPE within 30 minutes after completion of each training session.
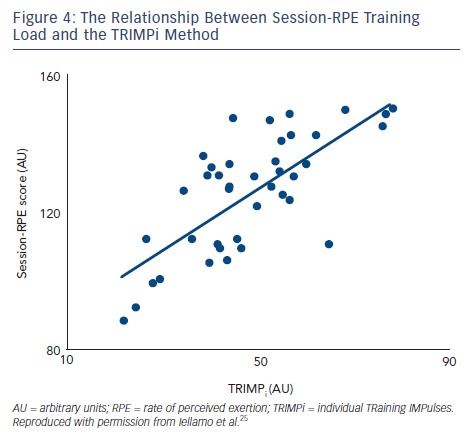
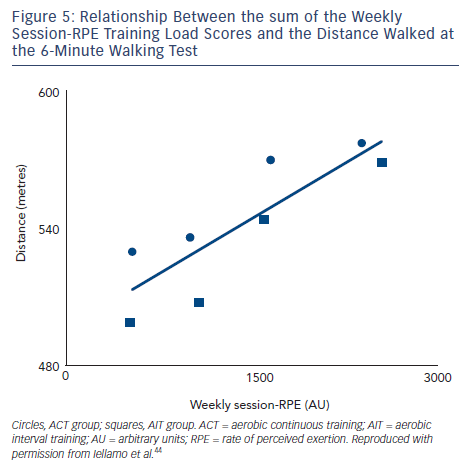
To assess the feasibility and reliability of the session-RPE method in patients with CHF, we compared functional capacity and ANS adaptations to session-RPE and TRIMPi methods, for both ACT and AIT in the same patients.44 The TRIMPi and session-RPE-based training loads were calculated from 672 training sessions and individual correlations were determined from 42 training sessions. Significant correlations were found between TRIMPi and the individual session-RPE, for both ACT and AIT, with r values ranging from 0.63 to 0.81; (P<0.05). Additionally, significant correlations between TRIMPi and session-RPE were observed when both ACT and AIT patients groups were pooled together (r=0.72; P<0.01) (see Figure 4). A progressive increase in the distance walked was observed with the increase in session-RPE score from the baseline throughout the study, as observed with the TRIMPi method.17,40 There is a significant linear relationship between session-RPE score and the distance walked at the 6MWT (r =0.85, P< 0.01) (see Figure 5).
R–R interval, HRV and BRS increased significantly with a rise in training load with both session-RPE training protocols. These were linked with a high weekly session-RPE score, with a second-order regression model resembling a bell-shaped curve in the ACT and an asymptotic-shaped curve in the AIT groups respectively. This was previously reported with the TRIMPi method.25
These findings strongly suggest that a simple method of training prescription/monitoring (i.e. the session-RPE) provides superimposable outcomes, in terms of functional capacity and autonomic adaptations, as those provided by an objective, HR-based method (i.e. the TRIMPi), in patients with CHF. The consistency of results during different exercise modalities (i.e. ACT and AIT) suggests that the session-RPE method may be useful for long-term prescription of varying exercise-based rehabilitation programmes in cardiac patients. From a practical point of view, to reach weekly effective training load (e.g. around 400 AU), an exercise training programme would need to comprise 4 days/week training with a session duration of 40–50 min at RPE score from 3–5 on the Borg 10-point scale.44 Session-RPE should not be viewed as a substitute for the current guidelines that use HR, VO2max or TRIMP methodologies for exercise prescription. Rather, session-RPE would add a further tool in the armamentarium of exercise prescription methods, particularly apt to long-term, out-of-hospital, physical activity of cardiac patients, with a large benefit also in terms of cost-effectiveness in comparison with HR-based training methodologies, especially in the elderly.
Preliminary findings45 suggest that the session-RPE may be used for long-term, post-hospital, physical activity management to improve and maintain functional capacity on the basis of a subjective, yet validated, tool. If confirmed in a larger number of patients, this information might have a huge impact on long-term exercise training prescription outside medically supervised settings.
Could Findings from TRIMPi Studies Translate into Positive Outcomes for Patients with CHF?
A recent editorial by Eijsvogels and Thompson stressed the relevance of the optimal dose of physical activity to prescribe in clinical practice.46 Some studies suggested that higher doses of exercise are not of greater benefit than moderate doses for reducing mortality47,48 and that a U- or J-shaped curve better reflects the association between physical activity dose and health. The findings ensuing from TRIMPi studies are in line, from a pathophysiological perspective, with these large longitudinal studies, in that they showed a non-linear dose–response curve between exercise training load and ANS cardiac regulation, with no improvement in HRV and BRS, and even a worsening of these risk markers at the higher doses of exercise, without any substantial benefit on functional capacity by a more vigorous physical activity. Clearly, our studies cannot provide answers on long-term clinical outcomes but, nonetheless, they could furnish some clues to this aim.
Conclusion
This review focused on the need for tailoring exercise training programmes to individuals, taking into consideration not only energy expenditure but the internal training load. Exercise prescription should not be solely based on VO2max or HR-derived parameters when a comparable metabolic load is intended, particularly in patients with CHF, although these have proven fundamental and highly useful in exercise training. The TRIMPi and the session-RPE methods described in this review represent a step forward in the characterisation of aerobic training tailored to the clinical and functional status of each individual within cardiac rehabilitation programmes. RPE-based training might be an extra stimulus to encourage a life-long, physically active lifestyle.
In CHF patients, the potential benefit of increasing exercise performance by increasing training load from moderate to higher doses of exercise should be weighed against the lack of an improvement in cardiac vagal modulation and the possible increase in the risk of adverse events. Further study is needed to determine the dose and mode of exercise training most effective in terms of musculoskeletal and cardiovascular complications and clinical outcomes in CHF patients.