Cardiovascular diseases (CVD) are a major cause of complications in pregnancies worldwide, and can be largely attributed to increased cardiovascular risk factors, such as obesity and hypertensive disorders.1 Today, up to 4 % of all pregnancies are complicated by CVD, with increasing frequency.2 Cardiomyopathies – whether inherited or acquired – represent the leading cause of maternal morbidity and mortality in Western industrialised countries.2 Among these, peripartum cardiomyopathy (PPCM) is particularly important because of notable foetal and maternal morbidity and a significant contribution to maternal deaths in previously healthy women.3–5 Early diagnosis and immediate initiation of an appropriate therapy are crucial in improving the prognosis of this life-threatening disease in young women.3,6–11
Definition, Epidemiology and Risk Factors of Peripartum Cardiomyopathy
The Study Group on PPCM of the Heart Failure Association (HFA) of the European Society of Cardiology defines PPCM as follows: “Peripartum cardiomyopathy is an idiopathic cardiomyopathy presenting with heart failure secondary to left ventricular systolic dysfunction towards the end of pregnancy or in the months following delivery, where no other cause of heart failure is found. It is a diagnosis of exclusion. The left ventricle may not be dilated but the ejection fraction is nearly always reduced below 45 %.”10 Other causes of heart failure, such as pre-existing cardiomyopathy, pulmonary embolism, amniotic fluid embolism, and myocardial infarction should be ruled out by thorough evaluation of the patient’s history, physical examination and by means of electrocardiography and/or cardiovascular imaging (such as echocardiography or cardiac magnetic resonance imaging).6,8 The course of the disease can range from mild forms with unspecific symptoms, such as exercise intolerance, general discomfort and peripheral oedema, to severe forms with cardiogenic shock, including agitation, orthopnoea and lung oedema.8 Increasing awareness and better diagnostic and therapeutic insights have contributed to improved outcomes in PPCM patients over recent years.11–15 If treated according to published recommendations, approximately 50 % of all women recover fully (defined as left-ventricular ejection fraction [LVEF] ≥50 % and New York Heart Association [NYHA] functional class I) whereas an additional 35–40 % recover at least partially (defined as improvement of LVEF ≥10 % and at least one NYHA functional class).12 Delayed diagnosis can negatively influence prognosis in these previously healthy women.
The incidence of PPCM differs widely depending on the ethnic and regional background of women. Interestingly, Africans and African Americans are at a higher risk for developing PPCM, with an estimated incidence of 1:100 in Nigeria, 1:299 in Haiti and 1:1,000 pregnancies in South Africa. Estimated incidences in Caucasian populations range from 1:1,500 in Germany to 1:10,149 in Denmark.4,10,12,16 An increase in incidence rates has been observed in the US in recent years. While the incidence was formerly reported to be one in 3,250 pregnancies, current data estimate an incidence of one in 1,150 pregnancies.15,17 This trend is also observed in Germany. This may be explained by rising maternal age, a higher number of fertility-assisted treatments and the higher incidence of hypertensive disorders of pregnancy. Higher awareness may also contribute to the detection of more cases with mild-to-moderate LV dysfunction. An ongoing, worldwide, multicentre, observational registry as part of the EURObservational Research Program (EORP) was initiated by the Study Group on PPCM of the HFA.18 The aim of the registry (https://www.escardio.org/Research/Registries-&-surveys/Observational-reg...) is to further investigate epidemiological data, patient characteristics and disease-specific outcomes.
Biomarkers
Clinical characteristics of PPCM resemble those of dilated cardiomyopathy and shared genetic predispositions can be found in approximately 15 % of all cases.19 PPCM is considered an independent entity distinct from other cardiomyopathies.20 Defined biomarkers to distinguish between PPCM and other cardiomyopathies are still largely missing, but careful analyses of the PPCM pathophysiology have enabled the identification of a set of specific diagnostic and prognostic biomarkers.6 The most used and widely available biomarkers are natriuretic peptides (i.e. brain natriuretic peptide [BNP] and NT-proBNP) and – although not specific for PPCM – normal values can exclude acute heart failure immediately.21 Other more specific biomarkers such as interferon-γ (IFN-γ), microRNA-146a (miR-146a), and soluble fms-like tyrosine kinase-1 (sFlt-1) are still under investigation and have not been used in clinical practice yet.22–24 Mebazaa and colleagues recently demonstrated that the concentration of the pro-angiogenic placenta growth factor (PlGF) in PPCM patients and women with acute heart failure was higher compared with non-pregnant women.25 The sFlt-1/PlGF ratios were lower in PPCM patients than in normal pregnancies without PPCM. Further research is needed to evaluate the role of blocking sFlt-1 or miR-146a, which shows promising results in the experimental setting but has not been tested in women yet.
Bromocriptine
Based on the favourable outcomes in PPCM patients, bromocriptine treatment has been widely introduced into clinical practice in Germany.9,12,26,27 Bromocriptine is a semisynthetic ergot alkaloid that is a potent agonist of the transmembrane G-protein-coupled dopamine 2D-receptor and various serotonin receptors in the central nervous system.28 It is administered orally with a very low bioavailability of <10 % because of a considerable first pass metabolism. Bromocriptine is highly bound to albumin (90–95 %), metabolised via the cytochrome P450 system and mainly excreted by the liver.28 It has been successfully used in various diseases such as prolactinoma, galactorrhoea, type 2 diabetes mellitus, acromegaly and Parkinson’s disease for many years.28–31 Recent research supports and encourages the use of bromocriptine in PPCM because of its ability to block prolactin (PRL) release from the pituitary gland.9,12,26,32
Pathophysiology of Peripartum Cardiomyopathy and Experimental Data
A combination of increased oxidative stress during late gestation and in the early postpartum period, and high levels of the nursing hormone PRL have been shown to be an important pathophysiological aetiology of PPCM.4,5,10,24,33–35 Under several conditions that cause oxidative stress, cleavage of the 23-kDa PRL to a 16-kDa PRL fragment (also called vasoinhibin) is triggered by proteases, such as cathepsin D and matrix metalloproteinases. This 16-kDa PRL fragment has strong angiostatic, pro-apoptotic and pro-inflammatory effects and destroys blood vessels thereby restricting oxygen and nutrition supply to the heart, ultimately resulting in heart failure. This concept was first demonstrated in 2007 using a mouse model with a cardiomyocyte-specific knockout of the signal transducer and activator of transcription factor-3 (STAT3).34 Absence of STAT3 causes oxidative stress that in turn increases proteolytic enzymes leading to the generation of 16-kDa PRL from full-length PRL. Bromocriptine ultimately salvaged the myocardium from detrimental effects by blocking PRL release from the pituitary gland. Additionally, 16-kDa PRL induces the release of microRNA-146a in endothelial cells, which in turn has detrimental effects on both endothelial cells and cardiomyocytes and consequently negatively affects heart function.22 This work has paved the way for the introduction of bromocriptine in current clinical practice.
Bromocriptine: From Bench to Bedside
Initially, case reports were published postulating a potential effect on LV function and recovery of bromocriptine when added to standard of care heart failure treatment (SHFT) in acute PPCM.36,37 However, there was some harsh criticism in the face of this new treatment concept considering the impact of bromocriptine on top of SHFT. Two proof-of-concept studies suggested a positive effect of an additive therapy with bromocriptine in PPCM patients. Sliwa and colleagues performed a single-centre, randomised, open-label pilot study of women with newly diagnosed PPCM treated by either SHFT alone (n=10) or SHFT plus bromocriptine (n=10) for a total of 8 weeks.26 In short, additive bromocriptine treatment resulted in fewer deaths, fewer patients in NYHA functional class III and IV and fewer patients with persistent LVEF <35 %. Despite the drawback of a rather small cohort and open-label treatment, the results were encouraging and strongly supported experimental data in clinical practice.
In an analysis of the German PPCM registry from 2013, Haghikia and colleagues also demonstrated a beneficial effect of bromocriptine on outcome in women with PPCM.12 In total, 64 of 96 patients (67 %) were treated with bromocriptine. The number of patients with full recovery did not differ significantly between the groups. Nevertheless, a significantly higher number of patients were classified as improvers (59 of 64 patients; 92 %) in the bromocriptine group compared with patients not treated with bromocriptine (23 of 32; 72 %). The percentage of women experiencing adverse events in the overall cohort (heart transplantation, left ventricular assist device [LVAD] or death) was 9.4 % (9 of 96 women). It should be noted that significantly more women were treated with bromocriptine in the group that did improve during follow-up. Furthermore, there were significantly more patients treated with beta-blockers and angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs) in this group. These results further strengthen the beneficial role of the combination of heart failure medication and bromocriptine in PPCM patients.
Based on the experimental insights and the first promising clinical results, a German prospective, randomised and multicentre trial in patients with severe acute PPCM (LVEF ≤35 %) was conducted comparing a short-term regime (bromocriptine 2.5 mg once daily for 7 days) versus a long-term regime (bromocriptine 2.5 mg twice daily for 14 days followed by 2.5 mg once daily for additional 42 days).9 A placebo group was not permitted for ethical reasons. Of 140 patients assessed for eligibility, 63 finally underwent randomisation. The leading cause for exclusion was a LVEF >35 %. The change in LVEF after 6 months was defined as the primary endpoint and assessed by cardiac magnetic resonance imaging. LVEF improved in the short- and long-term bromocriptine group by 21 % and 24 %, respectively. The difference between both groups did not reach statistical significance. However, in a subgroup analysis of patients with very low LVEF (<30 %), there was a trend towards a better outcome in terms of LVEF in favour of the long-term bromocriptine treatment. Because of the lack of a control group the results of this subgroup (LVEF <30 %) were compared with a cohort not treated with bromocriptine. These data were extracted from the Investigation on Pregnancy-Associated Cardiomyopathy (IPAC) study, which systematically analyses PPCM patients in the US.15 LVEF at randomisation was 28 % (short-term regime), and 29 % (long-term regime) in the bromocriptine trial. In the IPAC study, baseline LVEF was 35 % overall. In an analysis of those women with LVEF <30 % enrolled in the IPAC study, 37 % of patients had an event or a LVEF <35 % at follow-up. In the bromocriptine trial, one of 37 patients (2.7 %) with an initial LVEF of <30 % did not improve and remained <35 % at follow-up. Of note, no patient experienced an adverse cardiac event in the bromocriptine trial. In the IPAC study, six patients experienced a total of nine major events (death, LVAD, heart transplantation).9,15 It should be noted that only one of 63 patients in the German bromocriptine trial and 30 of 100 patients enrolled in the IPAC study were of black ethnicity. This might have influenced the results as black ethnicity is considered a risk factor for poor recovery and adverse cardiovascular events.15,17 However, the pilot study in South Africa showed a highly favourable outcome in African PPCM patients treated with bromocriptine (10 % mortality and a higher recovery rate in the bromocriptine group compared with 40 % mortality and no recovery in the group not treated with bromocriptine).26 Likewise, the use of bromocriptine was associated with a low rate of relapse in subsequent pregnancies (20 of 34 PPCM patients with African origin).32 These data further support the notion that PPCM patients of African origin seem to benefit from the addition of bromocriptine to SHFT.
Role of Bromocriptine in Subsequent Pregnancies
The role of bromocriptine treatment in women entering subsequent pregnancies after an initial diagnosis of PPCM has been unclear until recently. In a retrospective analysis of 34 patients from Germany, Scotland and South Africa, the addition of bromocriptine to SHFT immediately after delivery was associated with an improved outcome compared with patients not receiving bromocriptine.32 While LV function was not different at the time of conception, LVEF was significantly lower at follow-up after delivery and at follow-up in patients who did not receive bromocriptine immediately after delivery.
Safety of Bromocriptine
Given that bromocriptine has a negative reputation regarding adverse effects, such as vascular, neurological or psychiatric disorders, safety concerns may arise. Recent research has revealed no evidence of serious adverse events of bromocriptine treatment when used for up to 8 weeks in dosages up to 20 mg daily. In the previously-mentioned randomised German PPCM trial,9 three of 63 patients (4.8 %) experienced adverse events possibly related to bromocriptine treatment. Venous embolism occurred in two patients and peripheral artery occlusion was diagnosed in another patient, all of them treated with the short-term regime. No serious adverse event was noticed in the long-term group that was treated for 8 weeks. Nevertheless, some case reports suggest a potential prothrombotic effect of bromocriptine in postpartum women.38,39 The Hannover group therefore used at least prophylactic anticoagulation in their randomised study and basically in all their PPCM patients treated with bromocriptine.9 Therefore, the importance of anticoagulation (at least prophylactic heparinisation) during bromocriptine treatment should be emphasised.
Treatment Concepts of Peripartum Cardiomyopathy
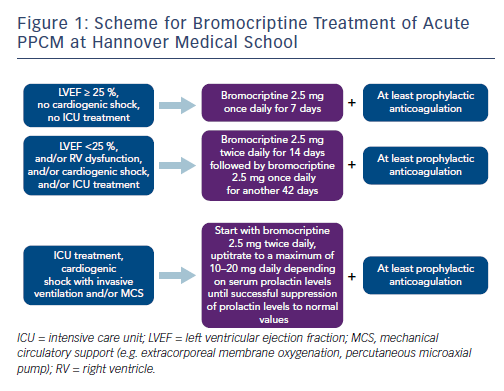
These results have led to the proposal of the so-called BOARD concept (Bromocriptine, Oral heart failure therapy, Anticoagulation, vasoRelaxing agents, and Diuretics) for the treatment of acute PPCM.27 Dose-adjusted bromocriptine should be applied to all PPCM patients depending on the severity of the disease. The bromocriptine treatment scheme of Hannover Medical School is depicted in Figure 1. Additionally, guideline-directed oral heart failure drugs should be initiated and uptitrated to the standard or maximal tolerated dosages in haemodynamically stable patients, including a beta-blocker, an ACE inhibitor/ARB/angiotensin receptor neprilysin inhibitor and a mineralocorticoid receptor antagonist. In acute PPCM with cardiogenic shock, bromocriptine should be added to acute heart failure therapy.8 Detailed treatment recommendations for heart failure patients are given elsewhere.6,8,21 Because of an increased risk of thromboembolic events, anticoagulation in at least prophylactic dosages should be initiated during bromocriptine treatment. In patients with systolic blood pressure >110 mmHg, vasorelaxing agents are recommended. Diuretics should be used in case of fluid overload.
It is important to note that the beneficial effect of heart failure and PPCM-specific therapy with bromocriptine may be considerably attenuated by the use of catecholamines such as dobutamine in patients with cardiogenic shock.8 Observations from the German PPCM registry suggest that patients treated with dobutamine had adverse outcomes, i.e. died or needed a VAD or a heart transplantation.40 Experimental analyses in mice confirmed toxic effects of beta-1 adrenergic receptor agonists in PPCM, showing that this treatment depleted the heart massively of energy and induced cardiac muscle necrosis.40 These effects are not influenced or salvaged by bromocriptine and/or the generation of 16-kDa PRL. As a consequence, a warning has been issued that catecholamines should be avoided in PPCM patients and alternative therapies, such as the inodilator levosimendan or temporary circulatory support devices should be used.8
In contrast to other forms of cardiomyopathies, such as dilated cardiomyopathy, most PPCM patients have a high potential for partial or even full recovery especially if early and optimal treatment as outlined above is applied. Suboptimal treatment may make the disease worse and can even lead to irreversible heart failure.
Conclusion
Taken together, the unique combination of basic, translational and clinical knowledge has led to a novel disease-specific treatment concept – the BOARD therapy regime – that reduces morbidity and mortality in PPCM patients. Treatment with bromocriptine is safe and effective and has contributed to improved prognosis in this still life-threatening disease.