More than 100 years have passed since the atrioventricular node (AVN) was first discovered by Sunao Tawara1 and described as a “Knoten” of tissue located at the proximal end of the Bundle of His (BoH).2 Despite the numerous advances in knowledge regarding the structure and function of the AVN, there are still several controversies that need to be addressed in both clinical and scientific settings.
The AVN is located in the paraseptal endocardium of the right atrium, at the apex of the Triangle of Koch, formed by the ostium of the coronary sinus, the tendon of Todaro and the tricuspid valve.3 Morphologically, the AVN can be subdivided into the lower nodal bundle and compact node (CN). From the lower nodal bundle, the rightward inferior nodal extension (INE) spreads along the tricuspid valve toward the coronary sinus and the leftward nodal extension spreads from the CN along the Tendon of Todaro.4
Similar to the sinoatrial node, AVN has the potential for pacemaker activity; however, there are significant differences between the activities of the two nodes. The normal AVN firing rate is 20–60 bpm compared with the 60–100 bpm of the sinoatrial node.5 One unique characteristic of pacemaker cells in the sinoatrial node and AVN is the process of diastolic depolarisation. An increased permeability to cations slowly depolarises the cell membrane. When the membrane potential rises and reaches the threshold for activation of the “funny current” (If), Na+ and K+ permeability increase, which then triggers an action potential (AP). The upstroke of the AP is mainly due to Ca2+ influx, as opposed to myocytes where Na+ plays a crucial role during upstroke. Finally, K+ efflux is responsible for repolarisation.6,7
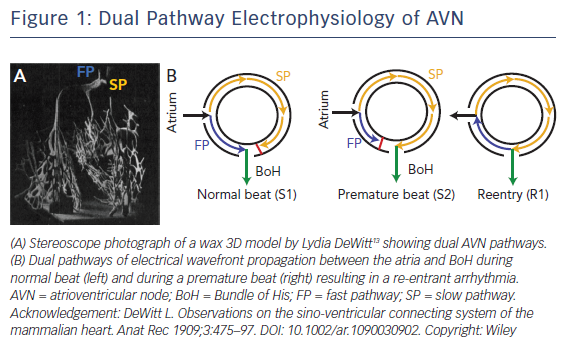
It has been well established that there are two functional conduction pathways in the atrioventricular junction: the slow pathway (SP) and the fast pathway (FP).8–12 The presence of these dual pathways was suggested as early as 1909, as evidenced by the dual AV nodal structure illustrated in DeWitt’s 3D wax reconstruction of the conduction system (Figure 1A).13 Anatomically, the SP is located in the INE while the anatomical substrate for the FP is less well defined. It is has been postulated to be in the transitional cell (TC) layers around the CN.14 Under normal conditions, excitation travels anterogradely through both the FP and SP. The wavefront through the FP reaches the BoH earlier than that through the SP due to the difference in anatomical distance and excites this region. The wavefront through the SP then reaches the refractory tail of the FP and is annihilated. This conduction pathway is illustrated in Figure 1B.15,16
Atrioventricular nodal re-entrant tachycardia (AVNRT) is the most common supraventricular tachycardia, accounting for greater than 50 % of all supraventricular tachycardias.17,18 It results from the formation of re-entry circuits between the AVN, at least two atrionodal connections or pathways, and a component of the atrial myocardium. The two atrionodal pathways, FP and SP, have historically been used to classify the type of AVNRT as SP-FP, FP-SP or SP-SP, depending on the direction of the re-entrant circuit and pathways involved.19 However, this classification could lead to confusion as these anatomical pathways are not consistent between patients. A newer approach to classify AVNRT was implemented by Katritsis and Josephson,18 depending on conduction times between the atria, BoH and ventricles. Briefly, AVNRT is classified as typical (SP-FP) or atypical (FP-SP, SP-SP). Most episodes of AVNRT will terminate with simple vagal maneuvres or pharmacological agents such as adenosine or non-dihydropyridine calcium channel blockers (verapamil and diltiazem).19 For chronic, recurrent arrhythmia, transcatheter ablation is a well-accepted technique that uses a minimally invasive method to ablate the lesions at the inferior or midpart of the triangle of Koch.20 In modern literature, this method has success rates of 93–98 % without recurrence.20–22 Many studies have identified the underlying molecular and structural causes for AVNRT and the efficacy of ablating the SP. This review summarises the structural, molecular and functional differences between the dual AVN pathways in humans.
Structural Heterogeneity
The components of the AVN are morphologically different in terms of cell shape, cell size and myofibril density. Of these factors, cell shape and size have previously been reported to alter electrophysiological variables such as conduction velocity (CV). Briefly, larger cells promote faster macroscopic CV through a given region and vice versa. The morphological heterogeneity in the AVN cells could partly account for the dual pathway electrophysiology.
Studies on human AVN cells have reported two cell types within the AVN and the surrounding tissue. The FP is composed of longer cells of larger diameter while the SP is composed of shorter cells that have smaller diameter. Additionally, the number of myofibrils in the SP closer to the coronary sinus is less and progressively increases toward the CN. The cells in the CN were also reported to show fewer striations relative to surrounding cells. In contrast, the BoH is composed of elongated cells.
Cellular morphology is also closely linked to intercalated discs that are cell–cell junctions with several important cellular coupling proteins. While the atrial myocytes (AM) and ventricular myocytes (VM) are composed of long, brick-shaped cells with many bifurcations and highly developed intercalated discs, the AVN cells have fewer bifurcations with underdeveloped intercalated discs.
Molecular Heterogeneity
Gap Junctions
Intercellular electrical coupling at the intercalated discs is achieved through gap junctions, which form pores that connect neighboring myocytes and allow for electrical and chemical communication between them. Gap junctions are hexameric protein structures that are formed by connexins (Cx) and their conductance varies depending on the Cx isoform.23–25 In the AVN, the most commonly expressed isoforms are Cx40 (large conductance) and Cx43 (medium conductance), followed by a relatively low-level expression of Cx45 (small conductance) and possibly Cx30.2/31.9 (ultra-small conductance).26 The AVN has a very complex yet unique distribution of these various Cx isoforms that facilitate its numerous functions. Several groups have studied Cx expression patterns in the human AVN.11,27,28 Total protein and mRNA levels of Cx40, Cx43 and/or Cx45 were assessed qualitatively and quantitatively in these studies. Representative Cx43 immunolabelled sections are shown in Figure 2B, alongside their serially sectioned histological images (Figure 2A).
AM and the BoH express both Cx43 and Cx40 proteins and mRNA in large levels. These Cx proteins are also expressed in TC, albeit at lower levels. The CN, however, has very high Cx40 expression with almost no Cx43. Penetrating bundle (PB) cells express large amounts of Cx40 but low levels of Cx43. INE also has high Cx40 expression; however, Cx43 expression in INE is different in the left nodal extension compared with the right nodal extension. Although the right nodal extension is usually longer in humans and has more Cx43, the left nodal extension is shorter (sometimes absent) and has very low C43 expression. The left nodal extension connects to the CN and forms a substrate for very slow conduction that could potentially sustain re-entrant arrhythmias within the AVN. Interestingly, the left nodal extension was also reported to be longer in pathological states such as dilated cardiomyopathy.11,27,28 The expression profile for Cx43 in the human AVN is shown in Figure 2C. To summarise, although Cx43 is highly expressed in the atrial and ventricular myocardium, its expression levels progressively drop off as we move into the central AVN region. In contrast, Cx40 is highly expressed in the AVN region and its expression levels drop off as we move away from the nodal cells to the AM and VM. Therefore, the expression profile of Cx40 is roughly opposite to that of Cx43. Finally, Cx45 and Cx30.2/31.9 are ubiquitously expressed at very low levels or are not present in the AVN.29
This heterogeneous expression pattern of Cxs within the AVN has several functional consequences. While on a larger scale, conduction slows within the AVN, resulting in AV delay, heterogeneous Cx expression is associated with dual-pathway electrophysiology. The dual pathways (FP and SP) paradoxically support relatively slower and faster conduction of electrical excitation, respectively. Conduction velocity is slower along the FP with lower Cx43 expression, and vice versa. Interestingly, higher Cx40 expression within the AVN does not correspond to faster CV. This could be due to a number of reasons, such as: (1) even though Cx40 protein is present, it may not form functional gap junction channels; or (2) conduction is further modulated by other ion channel protein expression and function.
Ion Channels
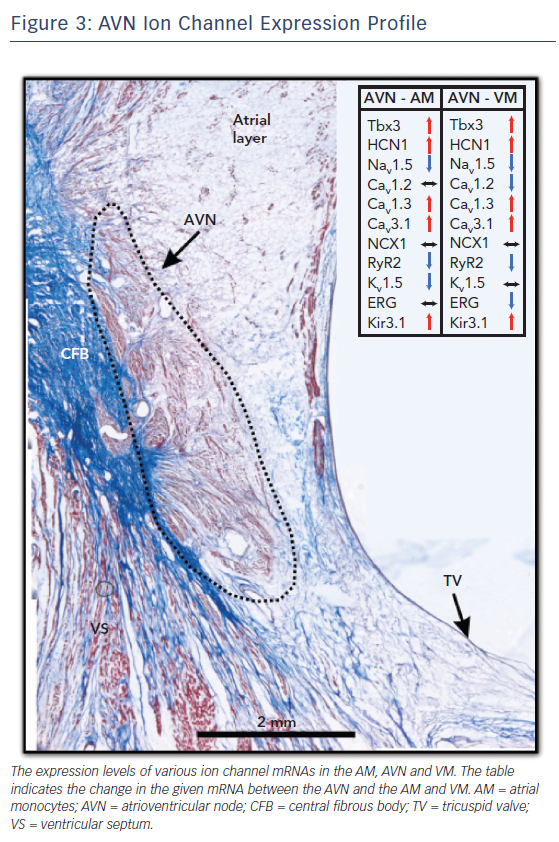
Each different cell type in the AV junction has its own electrical signature, that is, its AP. The AP morphology, duration and propagation are a result of the unique ion channel expression profile of the underlying tissue. The AVN, with its multiple cell types, is thus a collage of ion channel proteins and the expression profile of this complex structure is detailed below and summarised in Figure 3.
HCN Channels
The hyperpolarisation-activated channels or HCN channels are responsible for the “funny current” (If) in pacemaker cells and account for the automaticity of these cells.30,31 In the AVN AP, they contribute to the diastolic depolarisation phase. Four isoforms, HCN1, HCN2, HCN3 and HCN4, are present in the human AVN.32 Of these, HCN4 is the most abundant isoform. HCN4 mRNA was significantly higher in the CN, INE and PB cells relative to AM and VM, where they were mostly absent. HCN4 protein was also demonstrated to be upregulated in the CN and localised at the sarcolemma of these cells. HCN1 mRNA was predominantly expressed in the CN but its levels were low elsewhere. HCN2 and HCN3 were uniformly expressed throughout the myocardium and AVN cells; however, the latter was expressed at very low levels.27,28
Sodium Channels
Sodium channels are responsible for the fast upstroke of the AP and excitation of the myocytes. Several isoforms of sodium channels are expressed in the human heart and in the AVN region.33 Mutations in sodium channels result in AVN arrhythmias such as AVN block.34 Nav1.5 is the most abundantly expressed sodium channel isoform in the heart. The expression of Nav1.5 at the protein and mRNA level is high in AM and VM, very low to absent in the CN and INE and intermediate in the connecting layers of TC and PB.27,28 The neuronal sodium channel Nav1.1 is also expressed in the AVN cells and is most highly expressed in the INE and PB cells. It is uniformly distributed at lower levels through other cell types. Finally, studies have also reported low-level mRNA expression of Nav1.3, Nav1.4, Nav1.6 and Nav1.7.27,28 In the absence of a large sodium channel density in the AVN region, other ion channels such as calcium channels take over the cellular excitation and contribute to the upstroke of the AP.35
Calcium Channels
Calcium channels are the major drivers of depolarisation in the pacemaker cells of the AVN and sinoatrial node.35,36 The slow influx of calcium ions through voltage-gated calcium channels gives the AVN APs their characteristic slow upstroke. Two types of calcium channels are present in the AVN: L-type and T-type calcium channels. The expression profiles of their various isoforms are summarised in Figure 3. These channels are responsible for the L-type and T-type calcium currents (ICa,L and ICa,T), respectively.
Cav1.2, an L-type calcium channel, is the most abundantly expressed calcium ion channel in the AM and VM. It is also expressed in the AVN cells but at much lower levels.28,37 In the absence of sodium channels, it has been theorised that ion channels with a more negative activation threshold are required for cellular excitation. Cav1.3, another L-type calcium channel, meets this requirement.38 Cav1.3 mRNA is expressed at significantly higher levels in the human AVN, particularly in the INE, CN and PB regions, relative to AM and VM.27,28 Together, Cav1.2 and Cav1.3 contribute to ICa,L in the AVN.
Cav3.1 and Cav3.3 are the major T-type calcium ion channels in the human AVN. Cav3.2 mRNA was reported to be undetectable in the human heart.39 Cav3.1 is highly expressed in the AVN, particularly in CN, PB and INE cells, at levels significantly higher than in AM and VM.27,28 Cav3.3 has a more uniform distribution through the AVN region and its expression is also comparable to AM and VM.27,28
Calcium Handling Proteins
In the working myocardium, the primary purpose of calcium handling proteins in the cells is for facilitating contraction. However, this is not the case in the nodal and conduction system cells. It has been proposed that the purpose of calcium handling proteins in AVN (and sinoatrial node) cells is primarily to regulate the calcium clock and the pacemaking function of these cells.40 The level of sodium calcium exchanger isoform 1 mRNA was reported to be similar in AVN tissue relative to AM and VM; however, there was a slight upregulation in the CN and a small downregulation in the INE regions specifically.27,28 The sarcoplasmic reticulum calcium release and uptake channel expression was also previously investigated. While Greener et al.28 reported a lower abundance of ryanodine receptor 2 in AVN cells versus myocytes, Dobrzynski et al.27 reported an increase in ryanodine receptor 2 mRNA but a decrease in ryanodine receptor 2 protein in human AVN relative to VM. Ryanodine receptor 3 mRNA expression was higher in the TC and INE cells but similar elsewhere. Finally, the expression of the sarcoplasmic reticulum calcium pump SERCA2a mRNA was uniform throughout the AM, VM and AVN cells.27,28
Potassium Channels
Potassium channels are responsible for repolarisation of the cardiac cells. Based on the specific current they contribute to, they can be divided into three components: transient outward potassium current (Ito), delayed rectifier currents and inward rectifier current (IK1).41
Ito is responsible for the repolarisation during phase 1 of the AP. The major contributor to Ito in humans is voltage-dependent potassium channel Kv4.3.35,42 The expression of Kv4.3 mRNA in the human AVN is similar to the working myocardium. Other Ito contributors, such as Kv1.4 and Kv4.2, have significantly higher expression in the CN and PB relative to the working myocardium.27,28
Delayed rectifier potassium currents are ––of three types: ultra-rapid delayed rectifier (IK,ur), rapid delayed rectifier (IK,r) and slow delayed rectifier (IK,s). The ion channels associated with these currents are Kv1.5, human ether-a-go-go (hERG) and KvLQT (the LQT-like subfamily of voltage gated potassium channels), respectively.41,43 Kv1.5 mRNA expression was high in the AM but very less in the AVN cells and VM. hERG mRNA expression in the AM, AVN cells and VM were reported to be uniform by Greener et al.28 but Dobrzynski et al.27 reported that hERG mRNA expression was significantly higher in VM. Finally, KvLQT was uniformly expressed in AM, VM and all AVN regions except for the INE where it was significantly reduced.27,28
IK1 is responsible for maintaining the resting membrane potential (RMP) in most cardiac cells. However, the major IK1 ion channel, Kir2.1, is significantly reduced in the AVN, which could account for its more positive RMP.27,28,44,45 Other potassium channel isoforms responsible for IK1 are more uniformly distributed in the AM, AVN and VM.27,28
Autonomic Nervous System
Both sympathetic and parasympathetic nervous systems exert control over the AVN.16,46 Sympathetic innervation is achieved through the beta-adrenergic receptors (AR), which when stimulated can increase the rate of junctional rhythm in the human AVN.16 The mRNA of the two most commonly expressed beta-AR isoforms, beta1-AR and beta2-AR, are expressed throughout the AVN region at similar levels. The expression profile is mostly uniform between the AM, VM and AVN regions, except for a slightly lower abundance of beta1-AR expression in the PB and beta2-AR expression in the INE.27,28
Parasympathetic innervation in the human AVN is achieved by the activation of the acetylcholine-activated potassium channel current (IK,Ach). These channels are heteromers that are formed by the Kir3.1 and Kir3.4 potassium channel isoforms and are upregulated in the CN and PB cells.27,28 This could be an indication of the importance of parasympathetic stimulation of the AVN. Activation of the IK,Ach channels can then reduce AV junctional rhythm rate in the AVN.16
Thus, the AVN is under very tight autonomic control that can modulate its conduction and pacemaking properties, which determine AV delay and AV functional rhythm, respectively. Therefore, even though it is not the lead pacemaker in a healthy heart, autonomic control of the AVN is crucial to maintain normal conduction delays (<0.1 ms)
that allow sufficient time for ventricular filling and also prevent re-entrant arrhythmias.16,47
In addition to AV junctional rhythm rate, autonomic innervation can alter the lead pacemaker site within the human AVN.16 It has been reported that during control conditions, the intrinsic pacemaker site is at the proximal end of the BoH. When the AVN is treated with isoproterenol (sympathetic stimulation), the leading pacemaking site shifts to the CN region. This could be a result of the heterogeneous beta-ARs in this region. Similarly, when the human AVN is treated with acetylcholine (parasympathetic stimulation), the leading pacemaker site shifts to the TC area.
Functional Heterogeneity
The vast differences in molecular profiles of the AVN and its surrounding region underly the complex electrophysiological heterogeneities of the human AVN. The pacemaking capability, dual conduction pathways, refractory period variations and the other specialised functions and characteristics of the AVN stem from its unique structural and molecular composition. A few of these distinct features are discussed in detail here.
Action Potential
The differences in ion channel expression can result in differing AP morphologies in various compartments of the AVN. This morphological variation was observed by optical mapping of the human AVN.15,16 These AP morphologies closely match those recorded by patch clamping of the rabbit AVN, which also reported varying RMP and even major ionic currents contributing to the AVN AP.44 For example, while the AM and VM RMPs were more negative, the AVN cells had an RMP of around –50 mV. TC, which are intermediate cells between AM and AVN cells, had an RMP similar to atrial cells (–70 mV) whereas PB cells have an RMP closer to that of CN cells. These RMP variations closely follow the expression of the IK1 channels, which are responsible for maintaining a negative RMP. These channels are greatly downregulated in CN cells.
The maximum rate of rise of the action potential (dV/dtmax) was also different between these cells.44 The expression profile of sodium and calcium ion channels underlie this phenomenon. Specifically, in AM and VM, which have higher Nav1.5 expression levels and INa as the major depolarising current, a much higher dV/dtmax (80–100 V/s) was recorded. In contrast, in AVN cells with very low Nav1.5 and high Cav3.1 expression, ICa,L is the major depolarising current. This results in a small dV/dtmax (4–6 V/s) and gives the AVN cells their characteristic slow AP upstroke. TC cells had an intermediate dV/dtmax (22 V/s), possibly due to a mix of both types of currents.
Finally, AVN cells also had significantly shorter AP durations relative to AM and VM (113 ms relative to 155 or 215 ms, respectively).44 Phase 2 or the plateau phase was not very pronounced in these APs. AP duration heterogeneity was possibly due to the delayed rectifier potassium channel distribution, specifically hERG. However, TC and PB cells had AP durations closer to that of AM.
Refractoriness
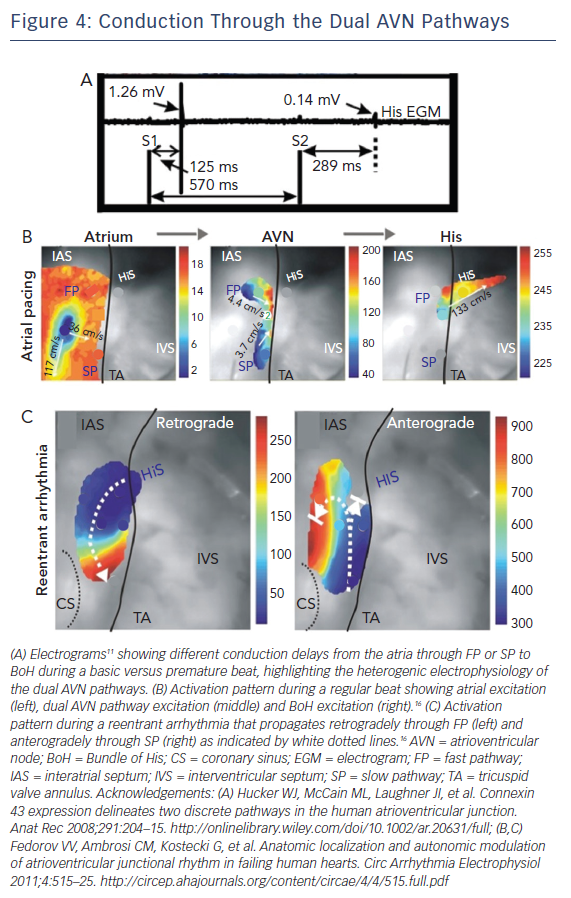
Another important electrophysiological difference within the different regions of the AVN is refractoriness or the time interval after an AP over which the cell cannot be re-excited. It has been demonstrated that the components of the SP have a shorter refractory period than those of the FP.48,49 One interesting result of this property was previously reported by electrograms recorded from the human AVN illustrated in Figure 4A.15 During atrial pacing using an S1S2 protocol, electrograms were recorded from the BoH. At shorter S2 intervals, the amplitude of the BoH electrogram was reduced and the delay between S2 and the recorded electrogram was increased. This indicates the switch in the conduction path from FP to SP at shorter pacing intervals, due to prolonged refractoriness of the FP. It also suggests the presence of two different compartments in the proximal BoH, which produce His electrograms of different amplitudes (FP: 1.26 mV versus SP: 0.14 mV).
Conduction Velocity
The AVN acts as the gatekeeper of electrical excitation between the atrial and ventricular tissue. Due to its unique ion channel and gap junctional expression profiles, the conduction of electrical excitation is slow in the AVN relative to the working myocardium.16 Additionally, there is CV heterogeneity even within the compartments of the AVN.10,15,16 The various molecular heterogeneities described above underlie these differences and give rise to the FP and SP of AVN conduction. During a normal beat, atrial excitation precedes AVN excitation as shown in Figure 4B (Left). The excitation wavefront then moves through the AVN, anterogradely through both the FP and SP (Figure 4B, Middle); however, excitation reaches the BoH earlier through the FP relative to the SP. This is then followed by BoH activation (Figure 4B, Right) and eventually ventricular activation.16 It is crucial to state here that the terminology of FP and SP does not refer to the CV through these structures. Paradoxically, the FP, which includes the TC and CN, is associated with slower CV relative to the SP which includes the INE. The terminology arises from the conduction delay through these structures. For example, even though CV is relatively faster through the SP, due to its increased anatomic dimension, it takes longer for excitation to reach the BoH through this pathway. Similarly, CV is slower through the FP but due to its shorter dimension, excitation reaches the BoH more quickly through this pathway.50
Arrhythmias
Abnormal activation sequences or rhythms through this complex nodal structure can result in the development of a re-entrant rotor within the dual conduction pathway of the AVN. This then gives rise to arrhythmias such as AVNRT.16 Optical mapping of the AVN during an SP-FP AVNRT episode is demonstrated in Figure 4C where the wavefront propagates retrogradely up the FP and then anterogradely through the SP. Other types of arrhythmias such as AV block can be a result of ion channel mutations.34 In these cases, the propagation of electrical excitation between the atria and the ventricles is either completely or partially blocked.
Summary
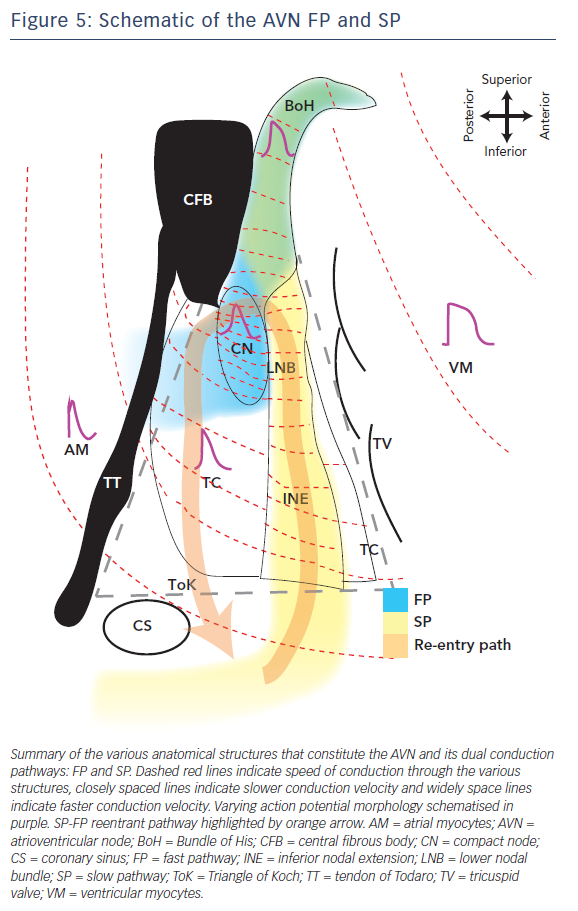
The schematic in Figure 5 illustrates the complex electrophysiological heterogeneities of the AVN, which include a dual conduction pathway involving varying CVs through the AVN and markedly different AP morphologies. The FP is the route of excitation wavefront propagation during a regular beat whereas the SP overtakes in the case of a premature beat or other AVN defects. This can then generate AVN arrhythmias such as AVNRT in which the excitation wavefront is trapped between the FP and SP and triggers excitation into the atria and BoH at a faster rate (tachycardia). This review highlights some of the key structural and molecular variants that underlie this complex electrophysiology and its predisposition to arrhythmias due to slight variations in normal activity. Concluding in the words of the poet Robert Frost, ‘I [conduction] took the road [pathway] less travelled by, And that has made all the difference [arrhythmia].’
Clinical Perspective
- Characterisation of atrioventricular node morphology will allow for the development of more efficient targeted pharmacological therapy for different types of arrhythmias.
- Better understanding of the electrophysiological pathways in the heart is crucial in developing precise diagnostic and ablation strategies.
- Identifying gene expression levels of these specific ion channels may allow for early identification of patients who are more likely to develop arrhythmias in the future.