The reported incidence of congenital heart disease (CHD) depends on the number of trivial lesions included, such as atrial and ventricular septal defects (ASDs and VSDs). Moderate-to-severe CHD numbers remain stable with 6 per 1,000 live births.1 Survival into adulthood has improved dramatically over the last 25 years and has been driven mainly by a decreased mortality in moderate and severe forms of CHD in childhood, including tetralogy of Fallot, truncus arteriosus, atrioventricular septal defect (AVSD), transposition of the great arteries (TGA) and univentricular hearts. For individuals born between 1990 and 1992 survival to the age of 18 was 78 % for tetralogy of Fallot, 77 % for AVSD, 71 % for TGA and 49 % for the heterogeneous group of patients with univentricular hearts.2
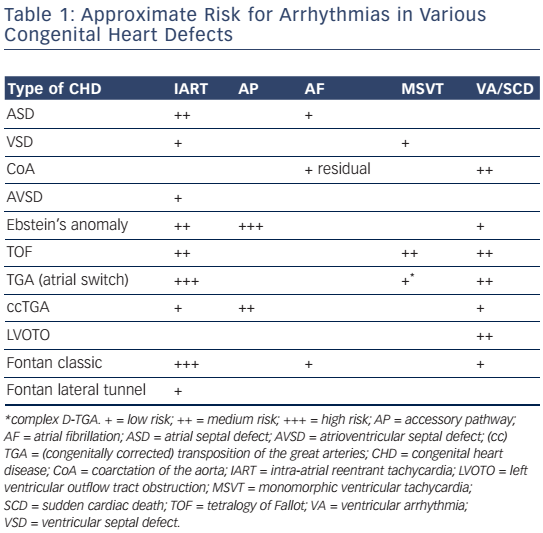
Improved surgical outcome during infancy increases the risk of late complications such as atrial and ventricular arrhythmias, thus contributing to morbidity and mortality later in life.3,4 Arrhythmias may be part of the underlying disease but may also be the consequence of the type of corrective surgery and the age at which it was performed (see Table 1).
Areas of dense fibrosis owing to surgical incisions as well as patch material, valve annuli and veins can form regions of conduction block that create anatomical isthmuses of myocardial bundles.5,6 Interstitial fibrosis due to longstanding cyanosis, pressure and/or volume overload, ageing and pathological hypertrophy may provide the substrate for slow conduction within these anatomically defined isthmuses.7,8 In particular, the duration of right atrial volume overload and right ventricular pressure overload have been associated with structural remodelling.7,9
The coincidence of both of these specific features – anatomical boundaries and pathological remodelling over time – in adult patients with CHD may explain the high incidence of atrial and ventricular reentrant arrhythmias.
Radiofrequency catheter ablation (RFCA) is an important therapeutic option for targeting these arrhythmias as drug refractoriness and unsuccessful antitachycardia pacing are often encountered.10,11 However, RFCA remains challenging due to the variable anatomy with occasionally difficult or limited access (occluded femoral vessels, interrupted inferior vena cava (IVC), surgically created obstacles, prostheses and baffles), the sometimes hypertrophied myocardium and a complex and highly variable arrhythmogenic substrate that is dependent on the original malformation and the type of repair that has been performed.8,12,13 The purpose of this review is to provide an update on the changing anatomical substrate found in contemporary patients with repaired CHD and its impact on catheter ablation.
Type and Timing of Surgery and its Impact on Atrial and Ventricular Arrhythmias
Surgical approaches and the timing of intervention in various types of CHD have changed over the past decades and are likely to affect the incidence and the potential substrate for arrhythmias (see Figure 1).
The first clinical surgical closure of an ASD was performed in 1948.14 Between 1948 and 1953 many methods of surgical repair were explored until Gibbons introduced the cardiopulmonary bypass in the mid-1950s. After its introduction, surgical ASD closure with direct sutures or with the use of a pericardial patch became widely accepted as the gold standard for ASD closure.14 Nowadays, minimally invasive surgical approaches using a limited oblique incision in the right atrium are increasingly used. Over recent decades, percutaneous device closure, which was originally described by King and Mills in 1974, has become an established alternative to surgery in selected patients with suitable anatomy.15
The arrhythmia burden after repair of ASD is substantial regardless of the type of closure performed, particularly when repair is performed during adulthood.16–19 In a large retrospective cohort of 213 patients after surgical ASD closure 2.3 % of patients developed new onset atrial arrhythmias and 60 % of patients with preoperative atrial flutter or fibrillation continued to have arrhythmias post-surgery after a follow-up period of 3.8 years.16 A retrospective study of 184 patients after percutaneous device closure demonstrated new-onset arrhythmias in 3.3 % of patients 4 months after repair.19 Silversides et al. reported an annual risk of new-onset atrial arrhythmias of 1 % in 101 patients after percutaneous ASD closure.18
The first intracardiac repair of tetralogy of Fallot was performed in 1954 by Lillehei on a 10-month-old boy. Subsequent series of early interventions reported a high perioperative mortality that led to the introduction of a 'two-stage repair' with a palliative shunt followed by total repair later in childhood.
Total repair included (patch) closure of the perimembranous or less frequently observed muscular VSD and relief of the infundibular or valvular right ventricular outflow tract (RVOT) obstruction. This repair was initially performed through a vertical or transverse right ventriculotomy often combined with the use of an RVOT or a large transannular patch to augment the restrictive RVOT. Right ventriculotomy and the use of a transannular patch with resulting pulmonary regurgitation and chronic volume overload often lead to right ventricle (RV) dilatation and dysfunction. Consequently, a combined transatrial–transpulmonary approach has been introduced, which is now usually performed early in life. The arrhythmia burden in repaired tetralogy of Fallot is considerable and increases markedly after the age of 45. The prevalence of AT was 20.1 % in a recent multicentre study, the majority classified as intra-atrial reentry tachycardia (IART).20 A significant number of patients had atrial fibrillation (AF), which is more likely in older patients with left ventricular dysfunction and left atrial dilatation. The vast majority of ventricular arrhythmias documented in repaired tetralogy of Fallot patients are monomorphic and fast ventricular tachyarrhythmias (VT) with a reported prevalence of 14.2 %.20 The modern approach of earlier repair using a transatrial– transpulmonary intervention is likely to have an important impact on the potential substrate and the incidence of ventricular arrhythmias.
The classic Fontan procedure, which is a direct atriopulmonary connection designed in 1971 for patients with tricuspid atresia has led to progressive systemic venous atrium (SVA) dilatation. With the aim of improving pulmonary blood flow it has been replaced by modifications to the procedure, which have also been applied to other forms of single ventricle circulation, such as hypoplastic right and left heart syndromes as well as other univentricular hearts.
One of the modifications is the lateral tunnel technique, introduced in 1988. It uses an intra-atrial patch to create a baffle to link the IVC to the superior vena cava (SVC), which is then connected to the pulmonary artery.21 The remaining morphological right and left atria serve as the pulmonary venous atrium (PVA). The extracardiac conduit-type Fontan described in 1990 consists of a direct extracardiac connection between the IVC and the right pulmonary artery using a Gore-Tex® conduit.22 The lateral tunnel has resulted in a significant decrease in late atrial tachyarrhythmias (AT) with a 15-year AT-free survival of 87 % compared with 61 % after the classic Fontan. If adjusted for the duration of follow-up the event-free survival was similar for the lateral tunnel and the extracardiac conduit Fontan in a recent paediatric cohort.23 However, considering the young population and the time dependency of an evolving arrhythmogenic substrate, a longer follow-up is required.
The Fontan operation and its modifications do not require ventricular incisions or patch material. Accordingly, monomorphic VT are unlikely and ventricular arrhythmias may be mainly attributed to impaired function and pathological remodelling of the systemic ventricle.
In dextro-transposition of the great arteries (D-TGA), there is a concordant atrioventricular (AV) connection in combination with ventriculoarterial discordance, with the aorta connecting to the morphological RV, and the pulmonary trunk to the morphological left ventricle (LV). It can occur in an isolated form (simple TGA) or in association with other congenital malformations (complex TGA: VSD in approximately 40 %, or less frequently in combination with pulmonary outflow tract obstruction). Senning performed the first atrial switch operation in 1959 using the atrial septum to create a baffle to redirect the blood flow from the caval veins to the LV. The RV remains the systemic ventricle. The Mustard procedure, introduced in 1964, uses a baffle from pericardium or synthetic material. Both procedures predispose patients to sinus node dysfunction and late AT with an incidence of 60 % and 24 %, respectively, at 20 years postsurgery.24 Simple TGA does not require incisions of the ventricles or patch material, which have been associated with monomorphic VT. However, in complex TGA, VSD patch closure, ventricular incisions and resection of outflow tract obstruction may result in anatomical boundaries facilitating macroreentrant VT as in patients with repaired tetralogy of Fallot.
Since 1975, anatomical correction with the arterial switch operation, first described by Jatene et al., has become the treatment of choice. During this procedure, the aorta and pulmonary trunk are disconnected from their arterial roots and 'switched' to connect to the correct ventricle.25 The prevalence of AT after the arterial switch remains low: in a reasonably large series only 3/65 (4.6 %) patients had a history of atrial flutter in adulthood.26 To determine whether the arterial switch operation also influences the potential substrate for monomorphic VT in complex TGA a longer follow-up is required.
Atrial Tachyarrhythmias
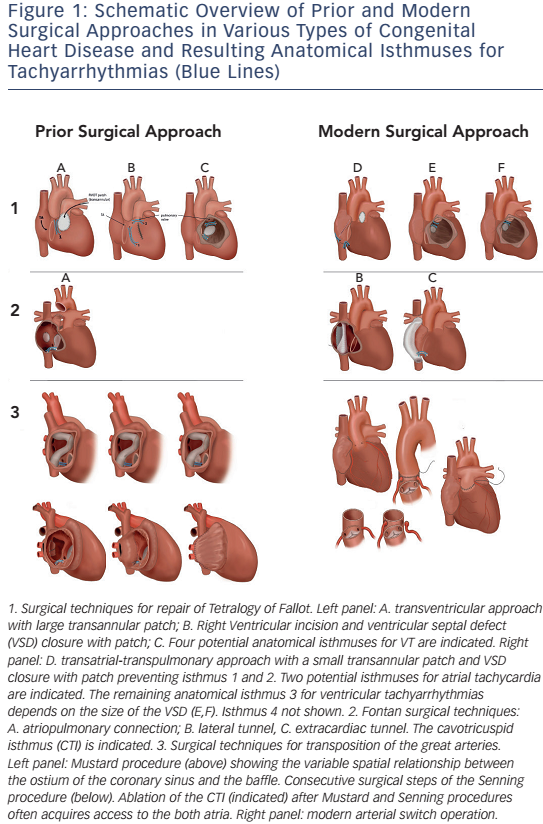
ATs are the most common arrhythmias observed in unselected patients with CHD, with a reported prevalence of 15 %.27 The frequency is highest after Fontan palliation and after atrial switch operation, followed by ASD closure and repaired tetralogy of Fallot (see Figure 2).28 Ageing plays an important role with a lifetime risk of 50 % once a CHD patient has entered the adult population.27
The dominant underlying mechanism is intraatrial (macro) reentrant:
- Cavotricuspid isthmus dependent flutter (CTIDF), which accounts for 40–60 % of all AT;
- Incisional IART using a suture line/prosthetic material as central obstacle, often involving the right lateral atriotomy (20–40 %), or, more rarely;
- Macro-reentrant or localised reentrant AT independent from these structures.29,30
Of importance, CTIDF and IART often coexist and are not distinguishable on surface electrocardiograms (ECGs).31 Considering the high incidence of CTIDF in patients with corrected CHD empirical cavotricuspid isthmus (CTI) ablation has been considered in noninducible patients.32
Although less frequent, non-automatic focal atrial tachycardias (FATs) do occur, accounting for 5–10 % of all AT.29,33,34 These non-automatic FATs are indistinguishable from macroreentrant by routinely applied noninvasive means. Foci are predominantly found in the SVA and may be due to microreentry or triggered activity.35 Body surface mapping is an interesting new technology, but whether it allows reliable differentiation between AT mechanisms in the presence of atrial scarring requires further investigation.
Mapping and Ablation
3D-electroanatomical mapping (EAM) to facilitate RFCA procedures has been successfully used for AT in patients with CHD.5,12,13,29,36,37 In patients with incessant or reproducibly inducible and haemodynamically tolerated AT, activation mapping can be performed to identify a focal source with a centrifugal spread of activation35 or to record the continuous sequence of activation covering the cycle length of a (macro-) reentrant AT.5 Zones of slow conduction can be identified and entrainment mapping can be used to confirm participation within the reentry circuit.5,31
Considering the complex and variable anatomy with multiple potential reentry circuits, a substrate-based approach facilitated by 3D mapping may be useful in selected patients. This method can also be applied in those who have an otherwise unmappable AT due to an inability to induce the clinically documented AT, multiple and changing circuits or haemodynamically poorly tolerated AT. The latter is more often encountered in patients with Fontan circulation or poor function of the systemic ventricle after the atrial switch operation. In particular the longer cycle length of IART may lead to 1:1 conduction and haemodynamic compromise (see Figure 3).5,38 3D voltage and activation mapping can be performed during stable sinus rhythm to identify boundaries of potential reentry circuit isthmuses. Sites with no atrial potential distinguishable from noise (generally ≤0.03 mV) and/or sites at which high output pacing (10 mA/2 ms) can be considered as dense scar or unexcitable tissue.5,39
Contact force measurement or catheter visualisation by intracardiac echocardiography (ICE) may be helpful to exclude poor contact and to identify true unexcitable scar. Delineation of unexcitable tissue may be important as far field electrograms up to 0.3 mV may mimic viable myocardium at sites with transmural fibrosis.40 Unexcitable sites can be displayed together with the caval veins, valve annuli and lines of double potentials. Double potentials may be located between the caval veins and the posterolateral SVA consistent with the crista terminalis but may also indicate atriotomy and suture lines.29,33 After delineation of all boundaries that create intervening isthmuses potentially related to AT, these isthmuses can be targeted by a linear RFCA lesion connecting the unexcitable boundaries during SR.
Considering the percutaneous access difficulties, particularly of the CTI in patients after complex surgical repair such as Fontan or atrial switch procedures, intra-operative transection of the CTI during initial repair could be a reasonable and desirable consideration in the future in selected patients.
Technical Developments
The use of an irrigated tip catheter has increased acute procedural success in patients with CHD.36,41 Long, steerable sheaths and real-time contact force measurement may be particularly helpful for differentiating between scar and non-contact sites in severely diseased and dilated atria after the classic Fontan. Whether this technique translates into more confluent and durable lesions and better outcome requires further studies.
Image integration (multi-slice computed tomography, cardiac MR) can facilitate ablation by visualising the complex anatomy and its relation to the catheter position. Late gadolinium enhancement cardiac MR may also be performed pre-procedurally to identify a potential arrhythmic substrate by visualising regions of myocardial fibrosis.42,43 However, whether anatomical isthmuses related to VT can be visualised by current magnetic resonance imaging (MRI) technology requires further evaluation.
ICE and its real-time integration with EAM may have additional advantages as it can facilitate real-time acquisition of the anatomy and landmark visualisation.38 ICE can also facilitate transseptal and baffle puncture and help to monitor tissue contact.13
Non-contact mapping allowing simultaneous recordings of >3,000 virtual electrograms has been successfully applied to elucidate the mechanism of right AT in a small group of patients, the majority after ASD closure.44 In patients with Fontan circulation who may benefit most from rapid mapping of multiple and often poorly tolerated AT, noncontact mapping was hampered by the size of the SVA with inaccurate scar delineation and appeared an inferior method to EAM.45 A promising new technology is high-density multi-electrode contact mapping with small and narrow-spaced electrodes. This is likely to facilitate substrate mapping by fast and accurate delineation of areas of slow conduction and small re-entry circuits in briefly tolerated AT.
Remote-controlled magnetic navigation (RMN) systems can potentially overcome navigation difficulties in CHD by providing improved catheter stability and manoeuvrability, reproducible catheter movement and precise delineation of cardiac anatomy.10,46 Irrigated tip RMN catheters and a fully integrated 3D electroanatomical system for superimposing EAM maps on the fluoroscopic reference images are now available.47,48 Use of RMN systems in combination with 3D electroanatomical systems has been reported to be safe and effective for ablation in patients with variable CHD complexities.10,46,48–50 Several studies suggest a significant reduction in fluoroscopy time using RMN, especially in patients with complex CHD.10,48,50 Whether RMN is superior to conventional mapping requires randomised trials that are unlikely to be available in the near future.
Endpoint of Ablation
Different definitions of procedural success and ablation endpoints have been applied and follow-up data acquisition varies between groups.
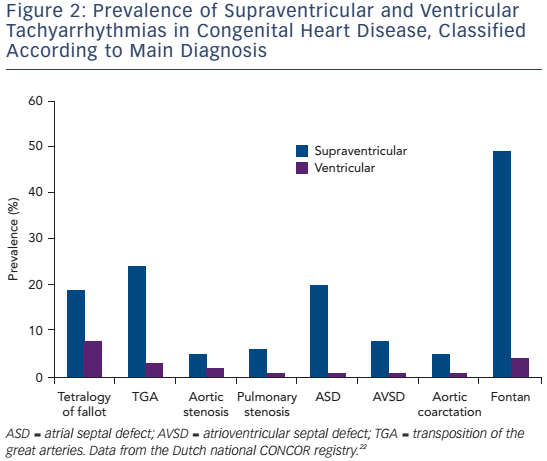
Accordingly, a wide range of acute procedural success rates (63–95 %) and outcome data have been reported. Outcomes are difficult to compare. They are dependent on patient population and the experience of the centres and operators. Table 2 provides an overview of the more recently published series.10,12,13,33,41,44,46,50–53 A bidirectional isthmus block is an established endpoint for CTIDF and has improved long-term outcome in patients after atrial switch operations.54 The procedural endpoint is less clear for IART for which termination during ablation is still often considered as 'procedural success' with or without non-inducibility at the end of the procedure.33,34,41,46,51,53 AT recurrence rates after RFCA are considerably high, ranging from 20–45 % at short-term follow-up. However, they can reach 85 % for complex CHD such as Fontan palliation, even if complete procedural success (defined as termination of all targeted IART and non-inducibility) has been achieved.53 The mechanism of AT recurrences may differ from the initial AT mechanism.12,13,33,37,46,51–53
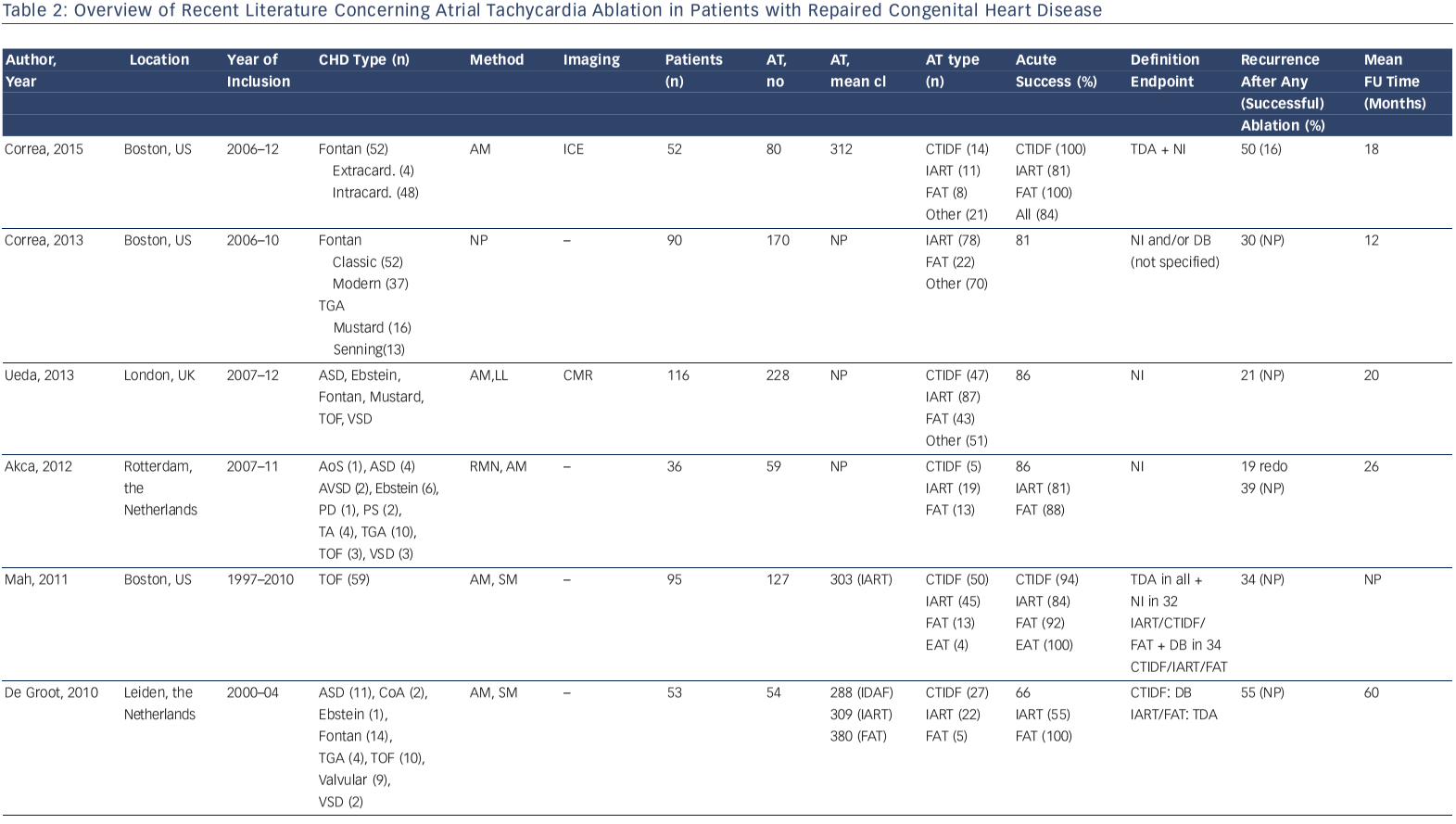
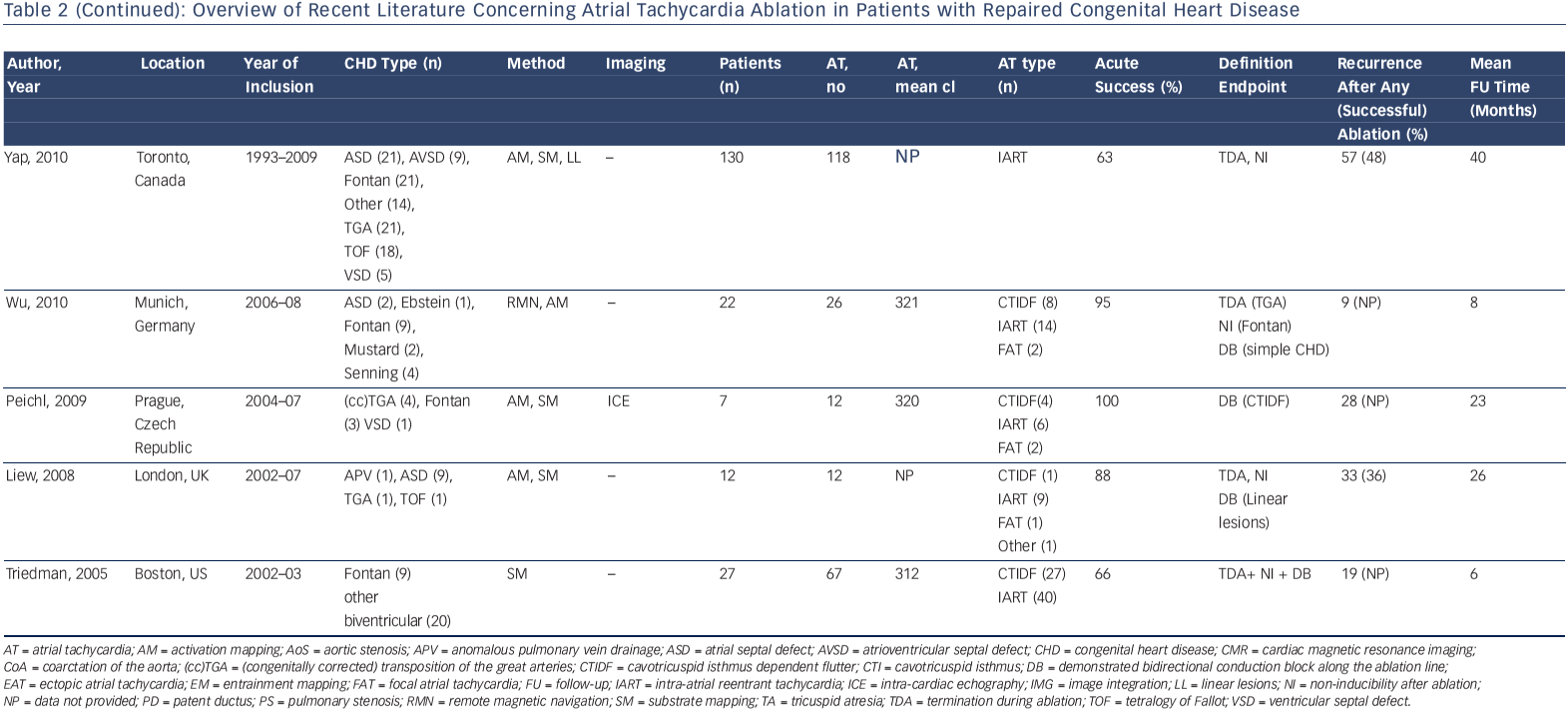

In a small series of 16 patients with various CHD all identified isthmuses based on electroanatomical substrate mapping were targeted, regardless of whether an AT was related to it. Unidirectional conduction block was applied as lesion endpoint. This approach was successful for 28 in 29 identified isthmuses. During a median followup of 14 months, only one new macro-reentrant AT was documented in one patient.5 The appealing concept of substrate-based ablation needs to be evaluated prospectively in a larger population.
Right Lateral Atriotomy
After right lateral atriotomy for ASD closure, repair of tetralogy of Fallot or as a part of many other corrective surgeries, the most common macro-reentrant circuit is a CTI-dependent flutter (52–71 %), followed by a macro-reentrant circuit with the lateral incision as central obstacle (21–31 %).29,33,34 Regardless of the initial ablation site >50 % of all recurrences occurred at either the CTI or the lateral SVA scar supporting an empirical linear ablation between the lateral SVA scar and the IVC.33
Fontan Circulation
After classic Fontan operation patients may encounter multiple IART circuits at various locations due to progressive SVA enlargement and time-dependent development of low-voltage areas that may serve as reentry circuit borders.5,55
CTI-dependent flutter (or cavomitral in L-looped ventricles) usually requires access to the PVA, either by a pre-existing fenestration, a trans-baffle puncture or, rarely, via a retrograde access.51 In patients with lateral tunnels, circuits may be localised to the low right atrium even in the absence of the tricuspid valve. Pericaval reentry is common with an area of slow conduction associated with the lower margin of the crista terminalis or low SVA surgical scars (e.g. cannulation site), which can often be terminated by focal ablation.56 Although pericaval reentry, which is typically slower than periannular reentry, can also be ablated by transecting the CTI-isthmus between the IVC and the hypoplastic or atretic valve, the latter may be more challenging due to an ambiguous anatomy and the risk of AV node injury.
In a series of 52 patients after total cavopulmonary connection, the majority of non-CTI-dependent reentrant circuits were located within the intracardiac baffle. It is important to note that focal AT can also be encountered and are often successfully ablated within the intracardiac tunnel.35,51,52
d-Transposition of the Great Arteries with Atrial Switch
The most important mechanism for AT in D-TGA patients after Mustard or Senning is CTIDF, accounting for 64–77 % of all spontaneous and induced AT. This is followed by IART and localised reentry, which may be confined to the PVA or SVA.29,30 The tricuspid valve and variable portions of the CTI are located on the pulmonary venous side, dependent on the surgical variant.57 In 81 % access to the CTI cannot be achieved via a systemic route but requires either a retrograde transaortic access through the AV-valve or a trans-baffle puncture (see Figure 4).54
Trans-baffle Access
An angiogram of the SVA can identify baffle leaks that may be crossed. In the absence of fenestrations or leaks, a trans-baffle puncture can be guided by fluoroscopy, transoesophageal echo or ICE with acute success rates of up to 96 %, if performed in experienced centres.58 In selected cases radiofrequency assisted transseptal perforation may be considered.59 In the extracardiac conduit Fontan transconduit access to the PVA can be challenging.
Ventricular Arrhythmias
The incidence of sudden cardiac death (SCD) in repaired CHD is 0.9–2.6 per 1,000 patient years, which is 25–100-fold higher than for the general population.60,61 Corrected cardiac defects often associated with ventricular arrhythmias and SCD are TGA, repaired tetralogy of Fallot and left sided outflow lesions (see Table 1). Cohort studies reporting on mortality cannot provide the type of ventricular arrhythmia leading to a presumed arrhythmic death.62 Ventricular arrhythmias encompass polymorphic VT, ventricular fibrillation in the absence of surgical scar and monomorphic VT; with a higher prevalence in patients who have undergone ventricular incision and patch closure of VSD. VT in repaired tetralogy of Fallot patients can serve as a paradigm for these postoperative monomorphic arrhythmias that are approachable by RFCA.
Indeed, based on ICD interrogation in patients with repaired tetralogy of Fallot and TGA, implanted for primary and secondary prevention, >80 % of all ventricular arrhythmias that prompted ICD therapy in repaired tetralogy of Fallot and around 50 % in TGA patients are fast and monomorphic VT often with heart rates >200 bpm.63,64 When rapid and untreated, these monomorphic VT may be fatal even in the presence of preserved cardiac function.
Moderate-to-severe systemic ventricular dysfunction is a dominant predictor for SCD in an unselected population of adults with CHD.54 Of interest, in a recent cohort two-thirds of early-to-middle-aged adult tetralogy of Fallot patients who died suddenly or experienced life-threatening VT had a preserved cardiac function before the first event.65 In addition, two-thirds of repaired tetralogy of Fallot patients referred for RFCA of fast and potential life-threatening, monomorphic VT also had a preserved biventricular function at the time of ablation.8 Whether more subtle parameters such as longitudinal LV function may help to identify patients at risk needs further study. Over the last decade progress has been made to determine the anatomical basis of monomorphic VT in repaired tetralogy of Fallot and complex TGA, which is crucial for both risk stratification and treatment.
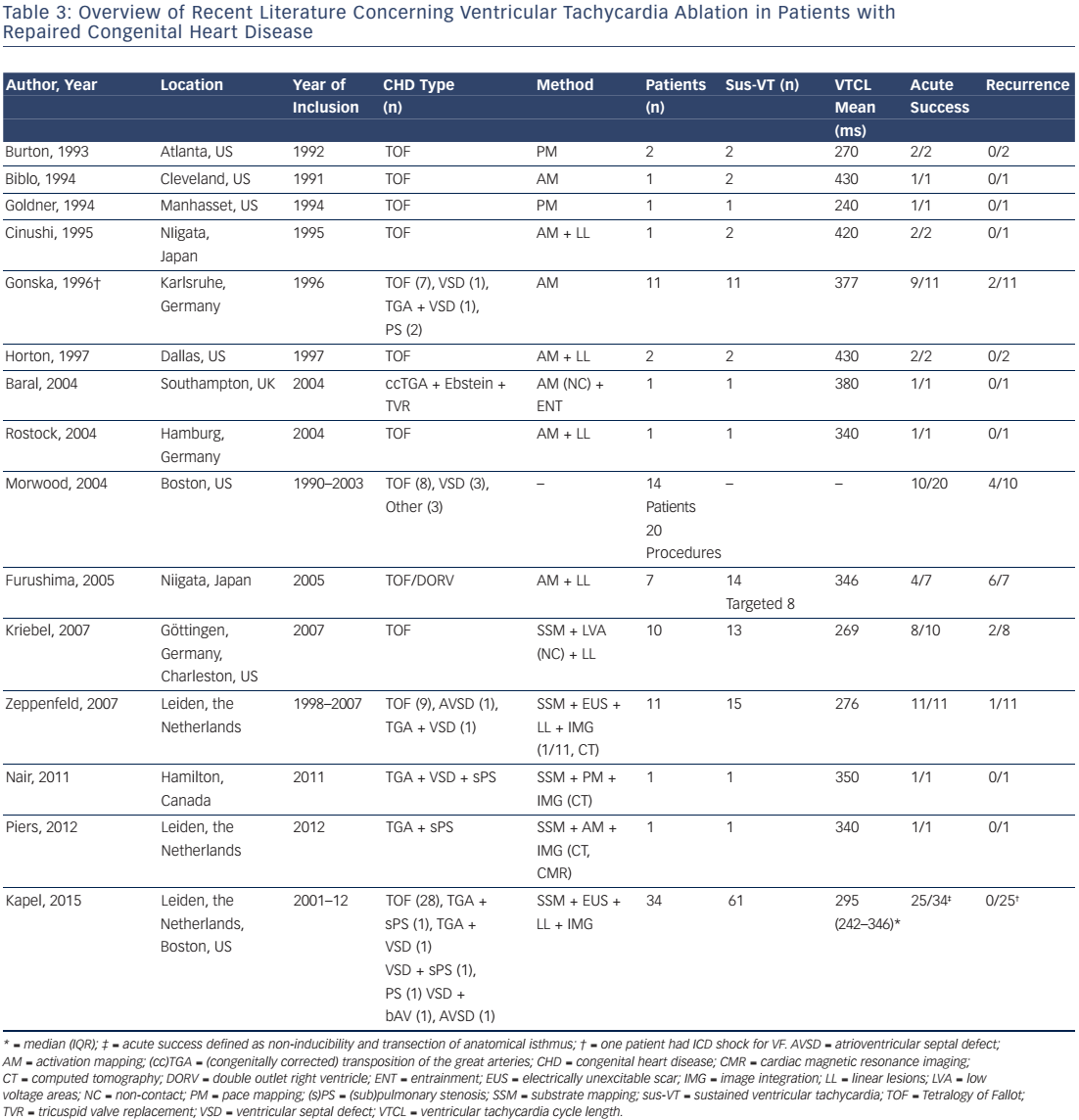
Anatomical Substrate for Ventricular Tachyarrhythmia and Impact on Catheter Ablation
The feasibility of catheter ablation of VT in patients after repair of CHD has been reported and Table 3 summarises the available data.6,8,66–78 The majority of treated patients were repaired tetralogy of Fallot patients. Accordingly, data on the mechanism and substrate of ventricular arrhythmias in other CHD is sparse.
Up to 97 % of all spontaneous and induced monomorphic VT in CHD patients referred for RFCA, or studied for risk stratification, are macroreentrant VT with a critical reentry circuit isthmus located within electroanatomically defined isthmuses.6,8,76 The majority of these VTs are fast and haemodynamically poorly tolerated requiring a substratebased ablation approach.6,8,76
Mapping and Ablation
A 3D reconstruction of all potential anatomical isthmuses can be obtained during sinus rhythm by identifying the boundaries, facilitated by the use of 3D mapping systems as described for AT. Bipolar electrograms >1.5 mV are considered normal ventricular voltage. At sites with amplitudes <0.5 mV, high-output pacing (10 mA, 2 ms) can be performed to identify unexcitable tissue, which is consistent with patch material or surgical scars. Depending on the malformation and the type of repair different anatomical isthmuses may be present. Four anatomical isthmuses related to VT in repaired tetralogy of Fallot have been identified (see Figure 1): Isthmus 1 bordered by the tricuspid annulus and the scar or patch in the anterior RVOT; isthmus 2 between the pulmonary annulus and the RV free wall incision or RVOT patch sparing the pulmonary valve annulus; isthmus 3 between the pulmonary annulus and the VSD patch or septal scar; and isthmus 4 between the VSD patch or septal scar and the tricuspid annulus in patients with muscular VSDs.6 Additional anatomical isthmuses have been described in complex TGA or after surgery for VSD and pulmonary stenosis.8,77,79
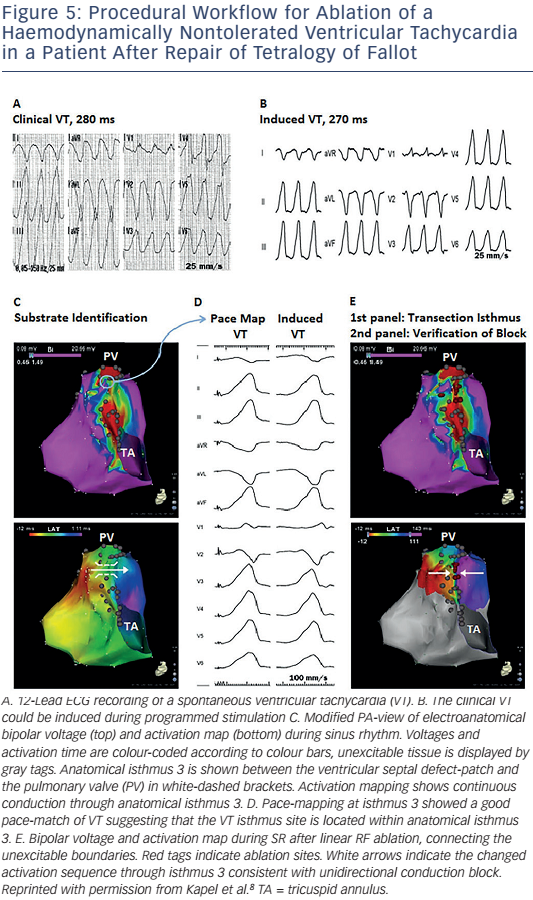
The critical reentry circuit isthmus of an induced VT can be determined by activation and entrainment mapping for haemodynamically tolerated VT or by pace-mapping for unstable arrhythmias.6,8 An alternative approach for mapping poorly tolerated VT is noncontact mapping. The system consists of a multielectrode balloon array and allows simultaneous acquisition of virtual unipolar electrograms. The activation sequence of fast VTs in 10 tetralogy of Fallot patients has been reported, the majority being macroreentrant circuits. The anatomical location of the critical isthmus could be identified in all patients.76 If the critical isthmus is located within an anatomically defined isthmus the anatomical isthmus can be transsected by connecting the adjoining anatomical boundaries by linear radio frequency (RF) lesions during sinus rhythm (SR).
Endpoint of Ablation
Demonstration of conduction block after isthmus transection provides a defined procedural endpoint similar to that for achieving block in the CTI (see Figure 5).8 A systematic substrate mapping and ablation approach has been applied in 34 adults with CHD (82 % tetralogy of Fallot). Complete procedural success was defined as noninducibility of any VT and transection of the critical anatomical isthmus, which was achieved in 25 patients. None of these patients had recurrence of a monomorphic VT during 46 ± 29 months of follow-up.8 These data suggest that macroreentrant VT based on an anatomical substrate can be treated effectively with catheter ablation. Isthmus ablation with confirmed conduction block should be attempted and, if successful, may be considered curative in patients with preserved cardiac function and no competing arrhythmia mechanism.
Arrhythmogenic Anatomical Isthmuses
Anatomical isthmuses are present in almost all repaired tetralogy of Fallot patients but not all are related to VT. Specific features of the anatomical isthmuses after initial repair, such as isthmus width and thickness of the myocardium, may be one decisive factor for further remodelling over time thereby forming the substrate for late VT.
In post-mortem series of repaired tetralogy of Fallot, isthmus 3 and 1 were present in almost all specimens, whereas isthmus 2 and 4 were observed in only 25 % and 13 %, respectively.6,80 In specimens from patients aged ≥5 years at the time of death, isthmus 3 was significantly narrower and thinner with more interstitial and replacement fibrosis than isthmus 1.80 In particular, narrow isthmuses with interstitial fibrosis may provide the substrate for slow conduction crucial for re-entrant VT.
To identify the characteristics of anatomical isthmuses that may serve as a substrate for VT, detailed electroanatomical voltage and activation mapping during SR has been performed in a cohort of 74 tetralogy of Fallot patients with at least one risk factor for ventricular arrhythmia, including 13 with prior documented VT. Narrow and slow conducting anatomical isthmuses (calculated conduction velocity <0.5 m/s) were the substrate for all 37 documented and induced ventricular tachycardia in 24 patients with preserved cardiac function (unpublished data). Considering the strong link between slow conducting anatomical isthmuses that can be identified and ablated during stable sinus rhythm and monomorphic VT in repaired tetralogy of Fallot, inducibility of the clinical arrhythmia and haemodynamic tolerance is no longer a prerequisite for successful ablation.
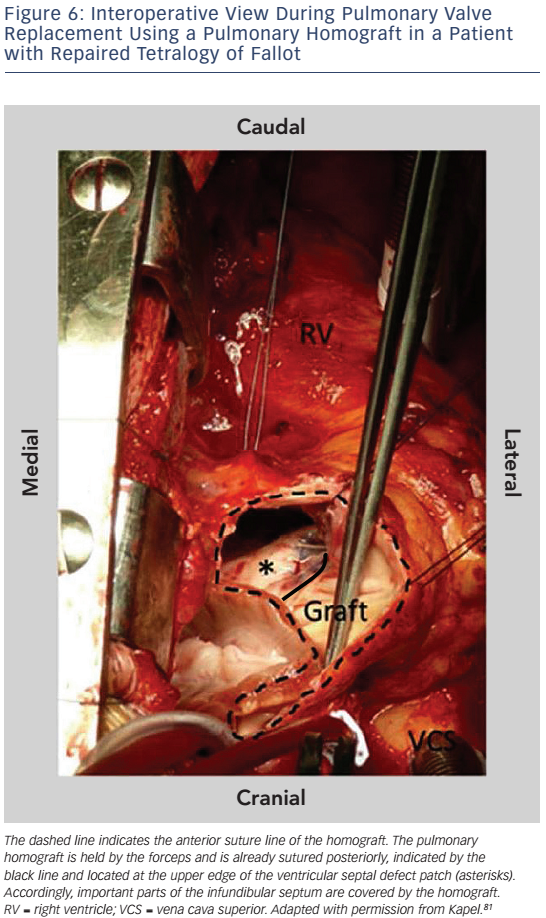
Important reasons for ablation failure are hypertrophied myocardium and protection of portions of anatomical isthmuses by patch material.81 In particular, in patients who have undergone pulmonary valve replacement (PVR) the implanted pulmonary homograft may cover parts of the infundibular septum preventing transection of anatomical isthmus 3 (see Figure 6). If ablation from the RV fails to transect the anatomical isthmuses a left-sided approach may be helpful for those anatomical isthmuses which involve the septum.81 The strong association between anatomically defined isthmuses, macroreentrant VT and the inability to transect isthmus 3 after PVR may justify pre-operative mapping and preventive ablation in patients with slow conducting anatomical isthmuses who require reoperation for pulmonary valve regurgitation.
With the introduction of a combined transatrial-transpulmonary approach and only limited patch augmentation for pulmonary valve stenosis, anatomical isthmuses 1 and 2 may be prevented in the majority of patients. However, isthmus 3 will remain. Evaluation of its potential arrhythmogeneity by EAM is appealing and could allow personalised risk stratification and tailored treatment for contemporary patients with repaired tetralogy of Fallot. Whether preventive transection of isthmus 3 during initial repair in the very young is feasible, reasonable and safe needs careful, multidisciplinary consideration.
Conclusion
With improved longevity for patients after repair of CHD, management of atrial and ventricular arrhythmias will continue to be of great importance. Patients with repaired CHD represent a highly heterogeneous group, displaying considerable anatomical diversity and complex, variable arrhythmogenic substrates. Enhanced understanding of the underlying substrate as well as technical developments for mapping and RFCA have improved outcomes in patients after CHD repair. However, continued focus on the understanding of the arrhythmogenic substrate in conjuncture with technical developments for the accurate delineation of its 3D anatomy and improved ablation techniques to achieve continuous and durable lesions is essential in order to further improve outcomes in this diverse population.
Clinical Perspective
- In patients with repaired congenital heart disease (CHD), improved longevity results in a high risk for late tachyarrhythmias leading to significant morbidity and mortality.
- Arrhythmogenic substrates in patients after repair of CHD have changed as a result of evolving surgical techniques.
- Radiofrequency catheter ablation remains challenging in patients with repaired CHD due to variable anatomy, surgically created obstacles and complex arrhythmogenic substrates.
- A substrate-based approach facilitated by 3D-electroanatomical mapping and accurate delineation of the anatomy is desired in catheter ablation procedures in patients with repaired CHD.