Treatment of atrial fibrillation (AF) classically focuses on eliminating triggers near and from the pulmonary veins, which may initiate AF. However, the 1–2 year success rate of pulmonary vein isolation (PVI) remains 40–50 % for persistent AF1,2 and 50–65 % for paroxysmal AF,3–5 while supplementary linear lesions or extensive ablation at electrogram-targets have had disappointing results and may not improve the success of pulmonary vein isolation.1,2,6 In recent years, focal or rotational drivers for AF have gained increasing attention as ablation targets. This approach is now supported by wide evidence ranging from optical mapping of animal and human AF7,8 (Figure 1) to several multicentre non-randomised clinical trials of AF driver ablation, yet the paucity of randomised trials in this area still remains a shortcoming.9–14
In this review, we hope to provide an overview of the mechanistic and clinical foundation of localised drivers of human AF, technical factors explaining why they may be revealed by some but not all mapping approaches, and potential explanations for why some clinical ablation studies have been disappointing despite promising results at many independent centres.
Mechanisms of Initiation of Human AF
Triggers such as ectopic beats,15 bursts of atrial tachycardia16 or varying autonomic balance17,18 may dynamically initiate AF, despite a static atrial architecture, including fibrosis.19 Dynamics in the physiology of atrial repolarisation and conduction can explain how triggers initiate AF. Ectopic beats, such as triggers from pulmonary veins, can produce dramatic oscillations of left atrial action potential duration (APD).16,20,21 The steep curve relating APD to diastolic interval (time between beats) leads to drastically shortened APD, lengthening subsequent diastolic interval, producing APD alternans and wavebreak, facilitating reentry and AF. These dynamics likely interact with atrial anatomy and/or fibrosis, explaining studies in which triggers dynamically formed spiral wave reentry at spatially conserved sites22,23 that initiated AF.
Mechanisms of Maintenance of Human AF
Once AF is initiated by triggers from pulmonary veins or other sites, two central hypotheses may explain how disorganised wave-fronts in AF are sustained. The multi-wavelet hypothesis shows disorganised activity that generates new wavelets in a stochastic fashion, such that no specific atrial region or structural element, e.g. fibrosis distribution is not critical to the maintenance of AF.24 In this hypothesis, extensive ablation should limit the critical mass for wave propagation and increase freedom from AF, although this has been contradicted by recent multicentre trials1,2,6 and by suboptimal results in some surgical studies that limit critical mass.1,2,6,25
An alternative hypothesis is that preferential regions of the atria can act as functional drivers for AF, manifest electrically in the form of spiral waves or focal activation patterns whose wave-fronts break down resulting in fibrillatory conduction. This hypothesis can reconcile the paradox of limited ablation terminating persistent AF in some patients,26–28 while extensive, untargeted ablation of left atrial regions (that may miss a driver region) being ineffective in others.1,2,29 This mechanism is supported by optical mapping, which remains the gold standard for mapping of fibrillatory conduction (Figure 1) and uses video imaging of voltage-sensitive dyes, coupled with phase, activation or other signal processing approaches to produce high spatial and temporal resolution maps of AF. Figure 1A illustrates rapid irregular action potentials at one anatomic site mapped optically. Figure 1B plots such action potentials across the cardiac surface in fibrillation. Each color represents phase (from activation to repolarization) such that rotations can be traced through the color spectrum (from red to blue, i.e. from early to late in the activation cycle). Points in the atrium where activation and repolarszation meet, i.e. around which an entire cycle can be traced, have undefined phase and are termed phase singularities (PS) which may represent rotor cores.30 Rotors are not fixed like reentry around an obstacle, but may precess in limited areas with complex trajectories (Figure 1C).31,32 This again distinguishes them from leading circle reentry, where the circuit is stabilised spatially around a depolarised core and which is not typically anchored to a region. Fibrillation driven by a rotational source may terminate when the source collides with a boundary, does not have enough “elbow room” to spin, or via other mechanisms.
Mapping AF: Applying Basic Science to Patients
Despite data in favour of rotational or focal drivers for human AF, there is an active debate on this issue with studies, predominantly using classical activation mapping, not showing any stable rotational drivers in AF.
This has highlighted a central issue in AF – that results and mechanisms may differ markedly depending on the mapping approach used. This differs from mapping of organised rhythms (e.g. atrial flutter), which yield similar findings regarding the underlying arrhythmia mechanism for different mapping methods. One specific difficulty with mapping fibrillatory waves is that an electrode antenna may capture several local and far-field waves. There are few means of separating these a priori except by knowledge of refractory period, and the traditional methods of visually ‘selecting’ local signals may be incorrect33 and alter reported mechanisms. Another difficulty is that activity changes in both temporal and spatial aspects rapidly within AF, which challenges mapping.
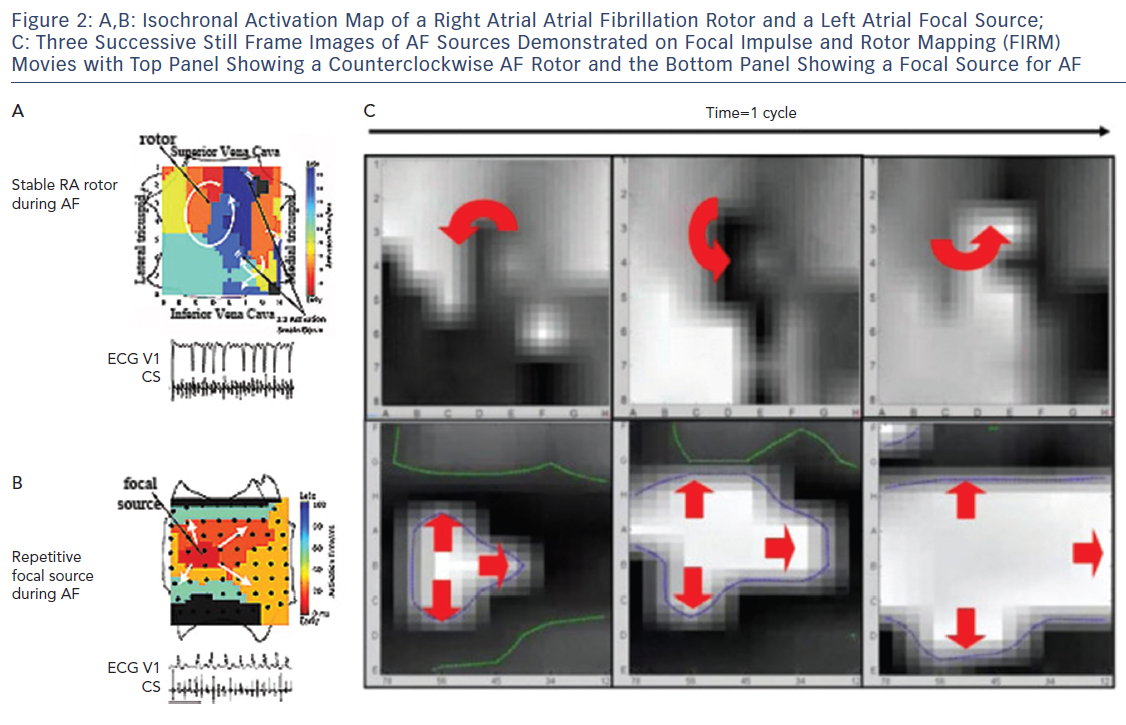
It became clear in early studies that unipolar, bipolar and MAP signals in AF differ dramatically,33 with examples of signals that appeared local in bipolar recordings actually being far-field, i.e. they lay within repolarisation indicated by the MAP.33 This may explain why optical maps of AF, which are based on local action potentials, show rotational drivers of AF in nearly all studies, while traditional electrode based AF activation maps mostly show only disordered activity.8,34,35 These findings, combined with data on conduction velocity and validation of mechanisms by patient-specific targeted ablation, led to Focal Impulse and Rotor Mapping/Modulation (FIRM).27,36,37 An example of a stable right atrial AF driver and a left atrial focal source is shown in Figure 2, where basket catheters are used to identify localised AF drivers. Theoretically, baskets can resolve 1–2 cm diameter reentry circuits in human AF as predicted by Allessie et al., and shown in human optical maps of AF.7,38 Conversely, if electrodes are too closely spaced to map fibrillatory conduction, calculated wave propagation may fall within measurement error. For instance, for atrial conduction velocity in AF patients of 40 cm/sec,39,40 reported errors in assigning onset time in AF (≈5–10 ms) translate to a distance of 2–4 mm (=40×0.005 to 40×0.010). Closer electrode separation than this may attempt to identify circuits within measurement noise, and will have less confidence. A related issue is that closely spaced electrode arrays typically cover small distances simultaneously, which may also miss the 1–2 cm diameter circuits in human AF, noted in optical maps.7,41
Reconciling Differences Between Mapping Studies of AF Rotational Drivers
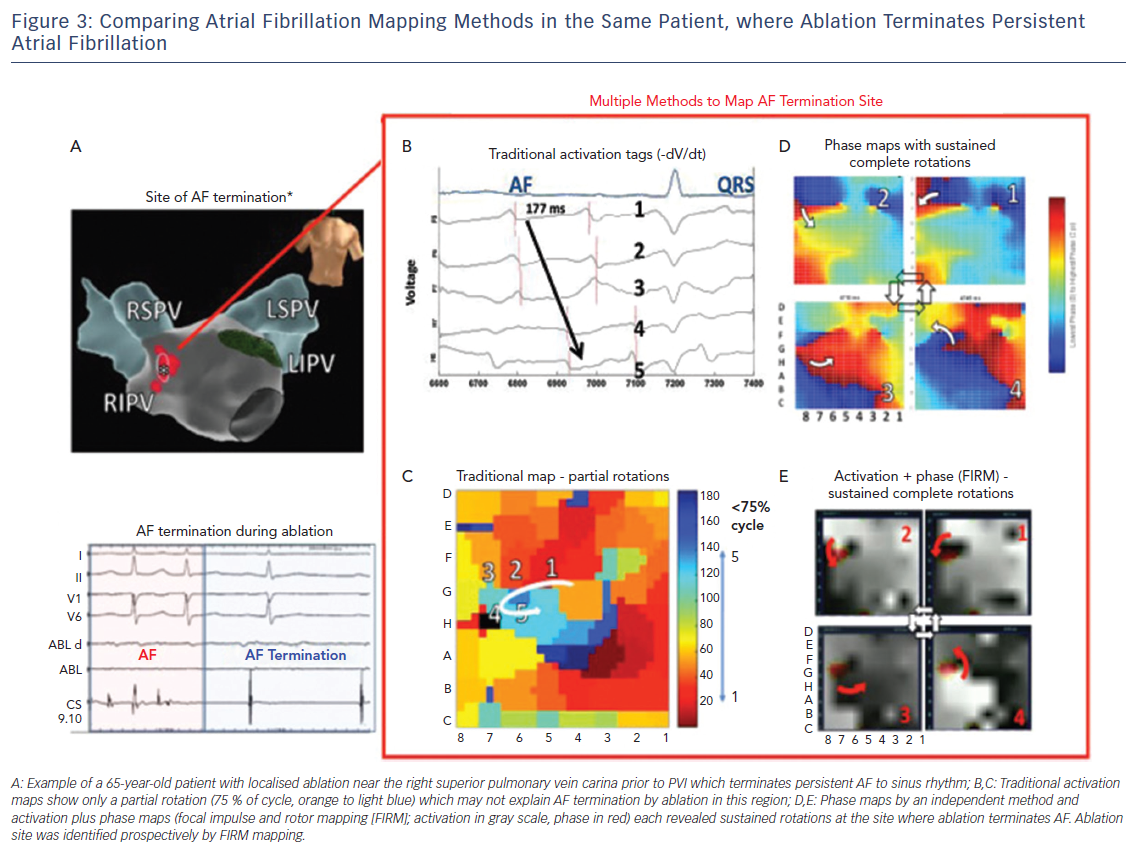
Since long-term ablation success varies dramatically between centres even for a well-defined, anatomic guided technique, such as, due to numbers of lesions, their durability, disease progression and other factors, reconciling differences in AF mapping may be assisted by other study designs.42 On the basis that sites where ablation terminates persistent AF may have mechanistic relevance, we have recently systematically compared maps created by different techniques for analysing the same raw electrographic data in cases of clear termination of persistent AF by ablation, as part of the international COMParison of Algorithms for Rotational Evaluation in AF (COMPARE-AF) study (NCT02997254).43
Figure 3 shows a patient in whom limited ablation before PVI terminated persistent AF to sinus rhythm. In panels C–E, detailed analysis of raw AF electrograms prior to ablation revealed sustained rotational activation by three methods. Of these methods, traditional activation maps of AF electrograms may be confused by spurious deflections, perhaps reflecting far field, and often showed only disorder at AF termination sites where phase and combined phase/activation maps showed rotations.
Long-term Outcomes of AF Driver-Guided Ablation
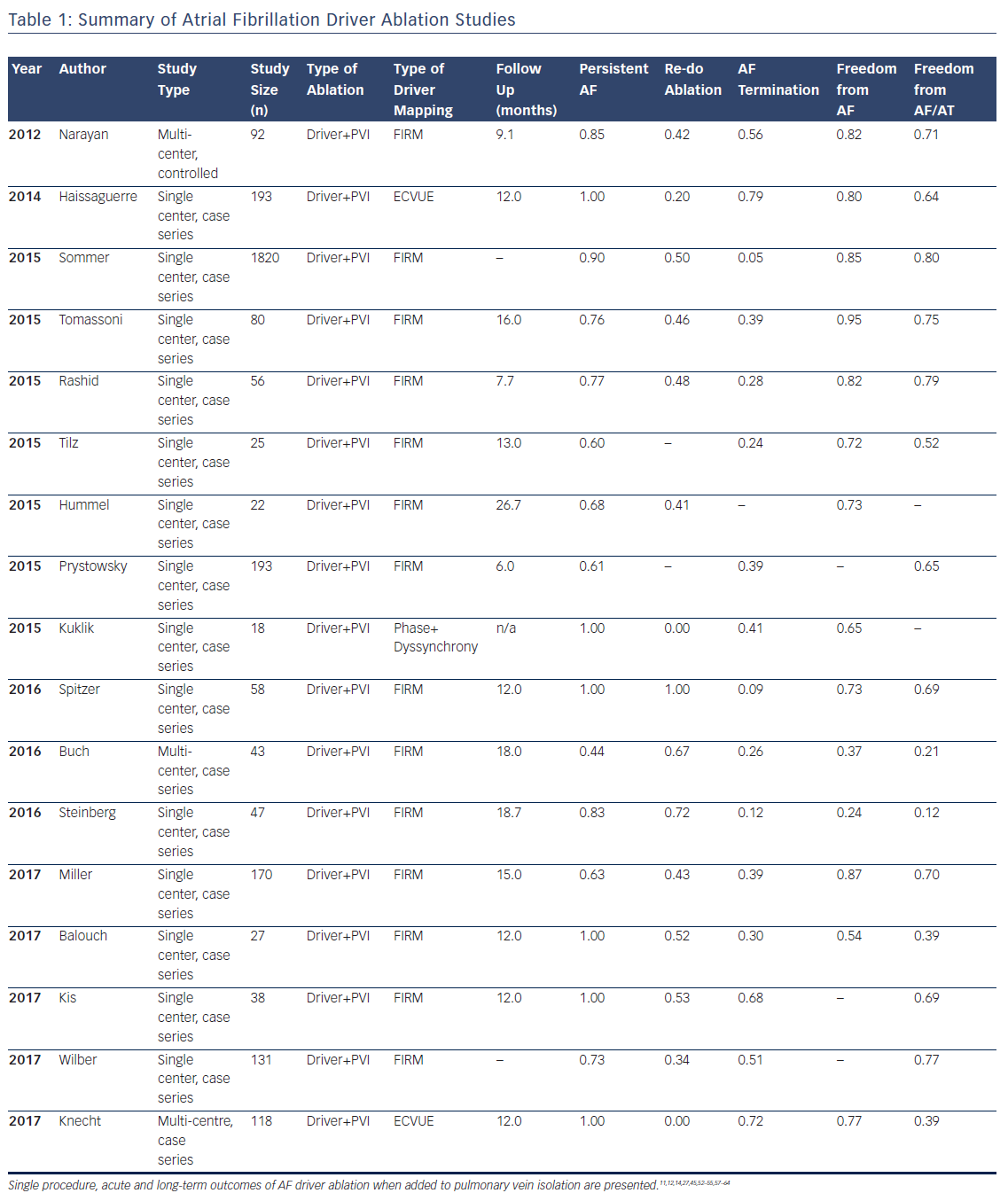
Multiple studies now exist on the outcomes of AF driver ablation (Table 1), with FIRM being the most widely applied technique. As with most new approaches, while initial reports were promising, some recent reports have been disappointing, such as Buch et al. reporting 21 % success and Steinberg et al. 12 % success. These results are surprisingly low since these series included many patients with paroxysmal AF and also performed PVI.44,45
Table 1 summarises studies in a total cohort of 1181 patients undergoing AF driver ablation in addition to PVI. Of these patients, 78 % had non-paroxysmal AF, and the overall single procedure freedom from AF and from AF/all atrial arrhythmias was 76 % and 62 %, respectively. It is noted that reading AF rotational/focal source maps and/or guiding ablation accordingly are novel skills and not learned as part of traditional PVI. Indeed, lower success was seen in studies listed in Table 1 that had fewer cases per operator, and in patients studied early (pre-2013) when no automatic tools were available to interpret maps to assist the operator in this early learning phase. Recent analyses confirm the incremental benefit of adding AF driver ablation to PVI, and there are several multicenter trials ongoing which may provide more clarity on this issue.46
In most studies, AF drivers arise in diverse locations, overall with 25–40 % near pulmonary veins, 25–40 % elsewhere in the left atrium, and 25–40 % in the right atrium. Body surface mapping (ECVUE) show similar AF driver distributions but in larger regions,11 which may represent greater ‘meander’ in projecting from the heart to the torso.47 In multiple studies, right atrial drivers are mostly in the free wall, posterolateral to the right atrial appendage, and rarely near the superior vena cava or cavotricuspid isthmus. AF drivers are present in higher numbers and more widely distributed in patients with persistent than paroxysmal AF.27
Atrial fibrillation rotational/focal driver sites have been compared to complex fractionated atrial electrogram (CFAE), and AF driver site areas are typically smaller than areas covered by CFAE. However, the concordance between AF drivers and CFAE differs between studies that may reflect differences in identifying CFAE or true differences between methods. FIRM identified sites do not show a specific CFAE grade or voltage fingerprint,27 i.e. CFAE arise at some AF driver sites but are prevalent elsewhere. Conversely, sites identified by body surface mapping could be related to CFAE.48 At the current time, electrogram markers for AF drivers are the subject of intense research.
Practical Approach in Ablation of AF Drivers
Core components required for effective driver-guided ablation include an effective broad-area mapping of both atria, precise identification of drivers to target, and ensuring full elimination of target areas. Each of these components is fertile for technical improvement, and may explain much of the heterogeneity in outcomes between centres.
The FIRM approach to widely map the atria uses multipolar contact basket catheters to analyze many wave-fronts at the same time. In the right atrium, the basket catheter is unsheathed in the superior vena cava (SVC) and slowly retracted into the right atrial body. Slight clockwise or counterclockwise torque is applied for optimal deployment. Particular care is required if a right atrial pacing lead is present, rotating the basket so that splines span the lead rather than displace it, and typically advancing from the inferior vena cava rather than pulling down from the SVC.
In left atrium, the basket is unsheathed in the left superior pulmonary vein and slowly retracted into the atrial body. Slight clockwise or counterclockwise torque may help achieve optimal basket deployment, maximising endocardial contact with the fewest splines over the mitral valve orifice. Alternatively, the basket catheter can be carefully ‘reflected’ off the carina of the left pulmonary veins.
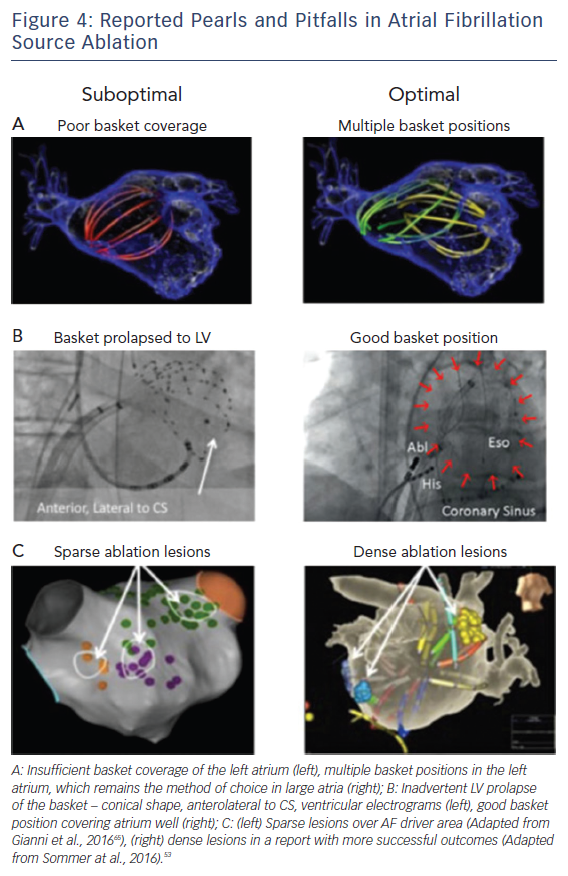
Figure 4 shows examples of optimal and suboptimal basket placement. Various basket sizes and types are available from an increasing number of vendors. We select the most appropriate basket size based on atrial size from intracardiac echocardiography or computed tomography. Figure 4B shows a suboptimal basket positioning that could be identified with fluoroscopic landmarks.49 Importantly, comprehensive atrial coverage may not be possible with a single basket position. Repeat maps after repositioning to sample previously poorly covered regions may help to reveal residual AF drivers.
Successful driver ablation should fully cover the affected driver areas, and contact force sensing catheters may help in this regard. Incomplete coverage may explain lower success rates in some AF driver ablation studies. For instance, Figure 4C shows sparse lesions that may not have eliminated drivers in the long-term, even if AF terminates acutely.50 Incompletely eliminated driver regions may cause recurrent post-ablation AF or atrial tachycardia (AT). Thus, it is critical to ablate the entire affected region, although the science on how much atrium to ablate has yet to be fully established.
Finally, AF driver-guided ablation does not appear to increase complication rates over traditional ablation alone,51 and is likely not pro-arrhythmic.12,44,52–56
In summary, the endpoint of AF driver ablation is the elimination of sustained rotational or focal activity that lie in conserved spatial locations. This endpoint is achievable in most patients, and may be superior to historical endpoints of AF termination. Lack of AF termination may reflect many mechanisms, including residual AF driver, but clinical results have been promising even in such patients if drivers are eliminated.
Limitations of AF Driver Mapping
While basic and translational science for AF drivers is mature, additional studies are required to reconcile technical differences in mapping for individual patient types. Clinically, large multicentre randomised trials are currently not available, but are ongoing. A learning curve exists in optimum basket placement, interpretation of AF maps, and ablation guidance based on maps. Each of these elements is a subject both for technical innovation and for clinical studies.
Conclusion
Evidence continues to mount that human AF is maintained by rotational and focal sources, and that targeting these areas may improve outcomes over PVI alone. We have outlined the scientific rationale for AF driver ablation, and practical strategies for ablation associated with promising outcomes in multicentre studies. Analysis of less promising studies suggests that suboptimal basket placement, resulting in greater difficulty in reading maps and in targeting ablation, and early learning curve experience may explain at least some of these discrepancies. Future improvements in AF driver ablation may be facilitated by better computational interpretation of AF maps, comparative studies between potentially complementary mapping approaches, and improvements in basket design. Combined mechanistic and imaging studies may enable better and functional classification of AF that may enable better patient tailoring of AF ablation. Ultimately, a better mechanistic and clinical understanding of AF may pave the way for novel drug discovery or regenerative therapies for AF. This is an exciting prospect.
Clinical Perspective
- AF drivers have been shown in animal and human optical mapping studies, as well as using several new mapping technologies.
- Studies demonstrate promising outcomes with rotor ablation when added to pulmonary vein isolation.
- Future improvements to the field would include better automated interpretation of AF maps, comparative studies between mapping approaches that may be complementary, and improved basket designs.