The rise and fall of interventional renal sympathetic denervation (RDN) as a treatment for arterial hypertension is a remarkable chapter in the scientific history of the 21st century. This once promising therapy has been abandoned by most clinicians after the neutral results of the Renal Denervation in Patients With Uncontrolled Hypertension (SYMPLICITY-HTN3) trial.1 It would have been easy to drop further efforts into the development of this technology, given this unsuccessful double-blind, sham controlled randomised controlled trial (RCT) of more than 500 patients.
On the other hand, as the incidence and prevalence of arterial hypertension remain high and patient adherence to medical treatment and lifestyle modification is, at best, suboptimal, the desire for a non-pharmaceutical treatment of arterial hypertension persists.
First, we will take a closer look at the history of RDN and specifics of the SYMPLICITY-HTN3 trial.
Interventional RDN is based on the paradigm of an elevated sympathetic activity in people with hypertension.2,3 The clinical relevance of this paradigm is supported by the blood pressure (BP) lowering effects of thoracolumbar sympathectomy in the first half of the 20th century.4,5 While the role of sympathetic overactivity in elevating BP is well established, this does not necessarily mean any interventional RDN is successful in any hypertensive patient. There is a constant proportion of patients in whom BP cannot be reduced by RDN, who are called non-responders. In almost every major trial on RDN, including the latest proof-of-principle Renal Denervation With the Symplicity Spyral™ Multi-electrode Renal Denervation System in Patients With Uncontrolled Hypertension (SPYRAL-HTN) and Endovascular Ultrasound Renal Denervation To Treat Hypertension (RADIANCE-HTN SOLO) trials, the rate of patients with a significant BP response (usually ≥5 mmHg change in daytime BP from ambulatory measurements) ranges between 60% and 70%.6–9 In early surgical trials, responder rates were even lower (45%), in spite of a marked reduction in mortality following treatment.5
The reasons for this non-response are not fully clear yet, but can be thought of as a mixture of three important factors.10 First, non-response can be explained by the absence of a substrate for RDN. Elevated sympathetic activity cannot be found in all patients with hypertension, especially elderly people.2,3 In this group, biomechanical components such as vascular stiffening and increased wave reflections might outweigh the sympathomimetic contributors to an elevated BP.11,12 Second, interference with antihypertensive medication, medication adherence and changes in treatment influence the effects of RDN on BP. Third, procedural factors, such as an unsuccessful or incomplete denervation procedure, could lead to a reduced or absent treatment effect. This final issue warrants thorough study to better understand the results of SYMPLICITY-HTN3 and of RDN in general.
Anatomical and Procedural Aspects of Renal Sympathetic Denervation
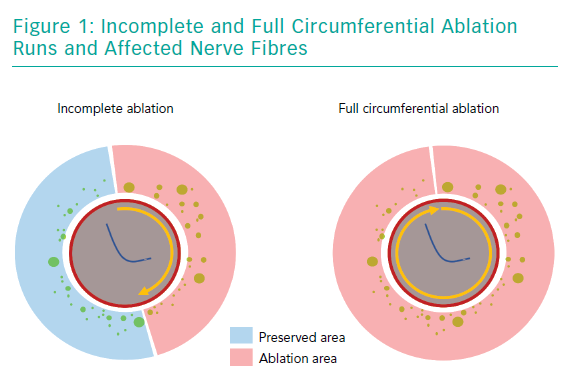
One frequently raised concern regarding SYMPLICITY-HTN3 was that it included a large number of centres with little practical experience in RDN. This is important, as to achieve a fully circumferential ablation pattern using a unipolar radiofrequency catheter requires some training and can be a challenge even for a skilled interventionalist. Achieving ablation of all four quadrants of the renal arteries is necessary to ensure complete destruction of the adjacent sympathetic fibres (Figure 1). Consequently, in a post-hoc analysis of the study, patients with a higher number of ablations had a more pronounced reduction in BP.13 These thoughts led to the development of newer RDN catheters that apply four ablations in a circumferential or spiral pattern using radiofrequency energy, simultaneous perivascular application of ethanol at three different points or even a fully circumferential ultrasound ablation pattern to facilitate the procedure itself.10,13–15
Notably, with all these devices, RDN is a black box treatment, as they do not allow direct feedback of treatment success to the interventionalist. In addition, the belief in the superiority of these newer generation catheters over previous technologies is based on technical considerations, but has never been proven in randomised clinical trials.
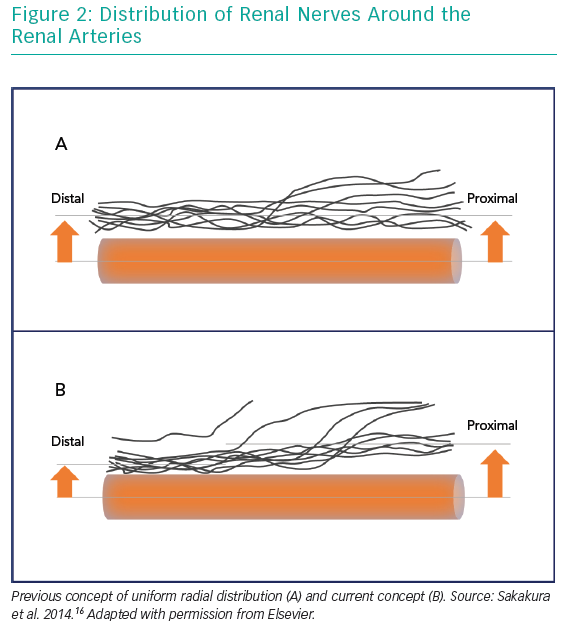
Another major paradigm shift following SYMPLICITY-HTN3 was related to anatomical considerations. Initially, RDN was carried out in the main renal arteries only. As it had been postulated that the distribution of renal nerve fibres along the main renal artery is uniform, it was assumed the exact location of the ablation points would not be decisive. RDN would affect the fibres at any point of the renal arteries’ course. A later anatomical study revealed that this assumption was wrong. Renal nerve fibres were not only found more frequently in the superior and ventral areas of the renal arteries, but were also closer to the vessel’s lumen in its distal sections and even more in its side branches.16
Therefore, ablation of the distal sections of the renal arteries and even side branch ablation might help to extend the ablation process and, ultimately, lead to a greater BP reduction. This hypothesis has been supported by animal studies.17,18
Notably, an additional ablation of the renal arteries’ side branches would require a longer procedural time and a greater volume of contrast agent. Side branch ablation would also require catheters that are small enough to enter these small(3–4 mm) vessels.
When using a denervation system that is small enough to reach into the renal side branches, it would be wise to use it on accessory renal arteries too. In the earlier SYMPLICITY trials, patients with accessory arteries were excluded, and a smaller retrospective study suggests a the effect of RDN is blunted when accessories are present.19
Another aspect that might have been underestimated in previous RDN trials is that the penetration depth of radiofrequency energy into adjacent tissue is limited to 3–4 mm.20 Particularly in the main renal arteries, this might not be deep enough to affect a significant number of sympathetic fibres.16 Therefore, increasing tissue penetration depth by using technologies with energy forms other than radiofrequency currents might increase the efficacy of RDN procedures. One such technology is the Paradise™ (ReCor Medical) ablation catheter. This catheter uses ultrasound energy to create a circumferential thermal ablation pattern. As the system is placed inside a cooling balloon, the directly adjacent vessel’s intima layer is protected, which allows a higher amount of energy to be applied. With this technology, an ablation depth of 6–7 mm into the tissue can be achieved, which theoretically affects around 90% of the adjacent nerves. Again, the superiority of this technology over previous approaches has not been proven in randomised trials. On the other hand, in a smaller trial in patients not responding to radiofrequency ablation, this technology significantly reduced BP.21
These considerations led to the design and conduction of the Global Clinical Study of Renal Denervation With the Symplicity Spyral™ Multi-electrode Renal Denervation System in Patients With Uncontrolled Hypertension in the Absence of Antihypertensive Medications (SPYRAL-HTN-OFF-MED) and Global Clinical Study of Renal Denervation With the Symplicity Spyral™ Multi-electrode Renal Denervation System in Patients With Uncontrolled Hypertension on Standard Medical Therapy (SPYRAL HTN-ON MED) studies and the RADIANCE-SOLO trial.8,9,22 In the SPYRAL-HTN-OFF-MED and RADIANCE-SOLO trials, drug-naive patients underwent RDN; the SPYRAL-HTN-ON-MED trial investigated RDN in patients taking 1–3 antihypertensive drugs. In these studies, the latest state-of-the-art ablation catheters and techniques were used by RDN-experienced interventionalists following a rigorous protocol. All three trials found RDN had superior BP reductions than a sham treatment.
Nevertheless, a direct head-to-head comparison of different ablation techniques and technologies was not available. This led to the design of the three-arm Randomized Trial of Different Renal Denervation Devices and Techniques in Patients with Resistant Hypertension (RADIOSOUND-HTN) study.23
RADIOSOUND-HTN
This trial was designed as a three-arm randomised controlled trial to compare the BP lowering effects of radiofrequency ablation of the main renal arteries using the Symplicity Spyral™ catheter (Medtronic) as considered reference standard with either an additional ablation of the renal side branches and accessories using the same device or an ultrasound-based ablation of the main renal arteries using the Paradise™ ablation system in people with therapy-resistant hypertension. To avoid the high variability and higher likelihood of regression to the mean from office BP measurements, ambulatory blood pressure measurements (ABPM) were used. The primary end point was mean change in daytime average from ABPM 3 months after the procedure. Patients’ inclusion and exclusion criteria were broad to allow a better transfer of the results to everyday clinical practice. The main inclusion criterion was resistant hypertension (systolic daytime BP >135 mmHg despite ≥3 different classes of antihypertensive drugs on at least 50% of maximum dosage) with stable antihypertensive drug treatment for at least 4 weeks. An additional key inclusion criterion was the presence of at least one larger renal artery (≥5.5 mm), because the anatomical considerations discussed above can be thought to be more distinct in larger vessels, so significant differences in treatment outcome would be easier to assess. Key exclusion criteria were being aged <18 or >75 years, having a life expectancy <6 months, pregnancy, secondary hypertension and any main renal artery diameter being <4 mm, as this would be the minimum diameter needed for treatment with the ultrasound balloon.
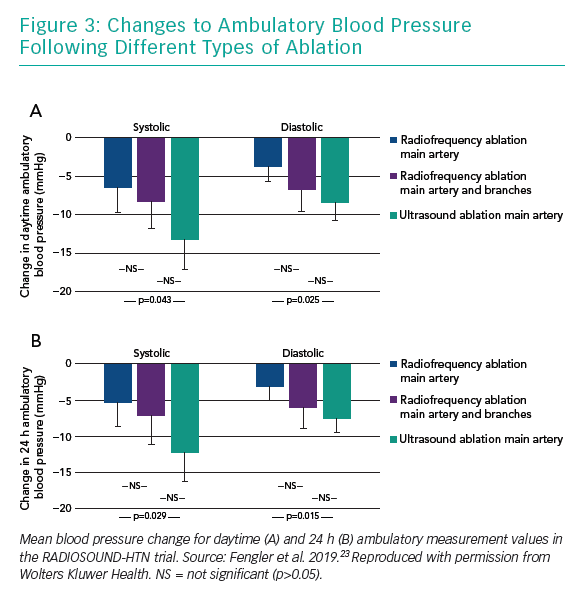
After 3 months, BP was reduced in all three treatment groups, with an average reduction of −9.5 ± 12.3 and −6.3 ± 7.8 mmHg for daytime systolic and diastolic ABPM values. The amount of BP reduction was significantly larger in the ultrasound ablation group than in the radiofrequency ablation group undergoing main renal artery ablation only (−13.2 ± 13.7 versus −6.5 ± 10.3 mmHg, mean difference −6.7 mmHg), while additional side-branch and accessory artery ablation was not superior to radiofrequency main artery treatment (−8.3 ± 11.7 mmHg for additional side branch ablation, mean difference −1.8 mmHg). Also, despite the numerical difference, ultrasound ablation was not found to be superior to combined main and side branch ablation in this trial (mean difference −4.9 mm Hg; Figure 3).
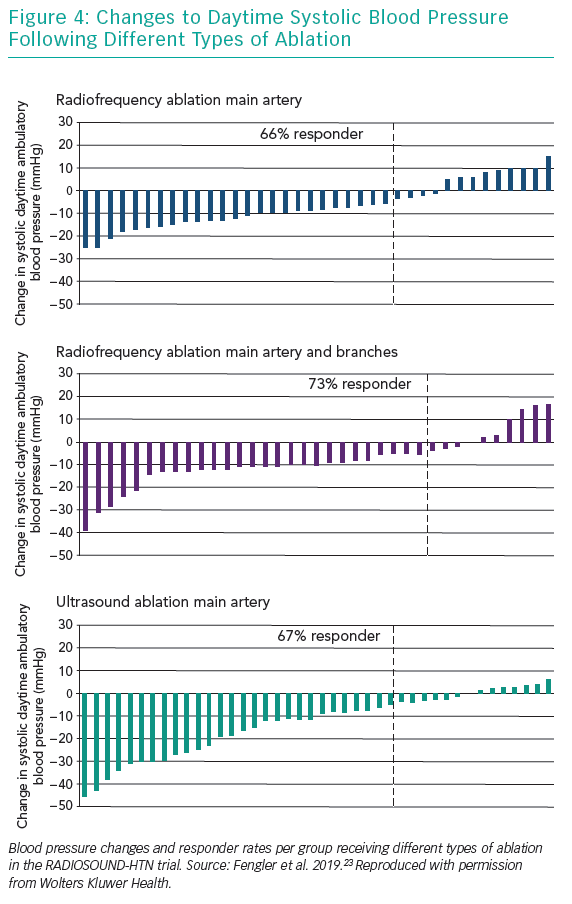
While the magnitude of BP change differed between the treatment groups, to our surprise the frequency of BP response did not (Figure 4).
Lessons from RADIOSOUND-HTN
Three major conclusions can be drawn from RADIOSOUND-HTN: First, there was a significant BP reduction in all three treatment groups. This underlines the overall efficacy of RDN in hypertension that was also found in the recently published proof of principle trials.8,9,22 Second, the use of newer ablation techniques and technologies seems to result in a greater reduction in BP but does not affect the frequency of patients not responding to the treatment. Third, while the ultrasound-based system was clearly superior to radiofrequency denervation when applied to the main renal arteries only, it is less clear if an additional side branch ablation would be beneficial.
The last finding is against expectations, as a previous non-randomised study and one randomised trial found additional side branch and accessory ablation was superior to isolated main renal artery treatment.24,25 Also, given the anatomical considerations, this more extensive ablation should have affected a markedly increased number of nerve fibres. On the other hand, this might be a cue for further investigation of renal nerve distribution and function: it is still not clear if ablation of afferent or efferent renal nerve fibres are key for RDN to work. Interrupting central afferences would reduce BP by downregulation of systemic sympathetic activity, and destroying central efferences could regulate BP by local renal diuretic and humoral function. It is likely that afferent renal fibres follow a different course to efferent ones.26 Possibly, ultrasound ablation of the main artery affects a different proportion of afferent and efferent fibres than distal and side branch radiofrequency ablation. Whichever of these fibres is the key effector of RDN, this might be one explanation for the diverging results in the treatment groups.
Another explanation for the non-superiority of side branch ablation over isolated main artery treatment is related to the specific treatment. While the ablation points were placed more distally in the main renal arteries than in previous trials and ablations were relatively extensive in this group, the number of ablation points in the combined main and side branch ablation group was numerically smaller than it had been in the SPYRAL-HTN trials.8,22
It will remain unclear whether additional side branch ablation is a useful extension of radiofrequency denervation until larger, adequately powered trials are conducted. In the meantime, investigators should consider whether, on balance, the increased amount of contrast agent and fluoroscopy times necessary for this procedure can be justified.
The observed BP responder frequencies in this study are counterintuitive. Our trial design was based on the assumption that more extensive ablation would lead to an increased number of BP responders, as fewer patients would receive an incomplete renal nerve ablation. This assumption was wrong. Instead, responder rates are comparable to those found in previous RDN trials or even below this level when applying the office BP response used in the first SYMPLICITY trials.7,27,28. As responder frequencies are not higher in the SPYRAL-HTN and RADIANCE-SOLO trials using the same treatment methods, a statistical outlier is an unlikely explanation.8,9,22. Instead, this highlights the importance of wise patient selection when enrolling for RDN trials.
It is likely that the rate of responders will not be improved unless only patients with an elevated sympathetic tone are enrolled, which is essential for RDN to work. In those patients, a more extensive ablation procedure will likely lead to a pronounced reduction of this overactivity, resulting in a greater lowering of BP. As other, non-invasive methods are not available, assessment of renal sympathetic activity requires invasive measurement of renal norepinephrine spillover. This is difficult to perform under the circumstances of clinical trials and will be even more challenging in clinical practice. In addition, norepinephrine spillover has never been investigated as a predictor for BP response after RDN. Therefore, future trials should focus on this issue. In the meantime, other potent predictors, such as elevated vascular stiffness could be used as surrogate markers for a biomechanical contribution to hypertension instead.11,12,29–31
Tasks for Future Trials
While improvement of the ablation technology itself might have already reached its peak, several open questions remain to be answered by future RDN trials. In particular, three topics will be of interest in the next few years. First, despite research in catheter-based RDN for decades, a direct feedback mechanism that can assure whether ablation was successful during the procedure is still lacking. This will be an important task for future research. Second, the role and anatomical distribution of afferent and efferent renal fibres for RDN need to be clarified. Third, the mechanism(s) by which RDN results in BP reduction is/are still unclear. Uncovering this secret will certainly open new possibilities to optimise patient selection and medical co-treatment.
All in all, RADIOSOUND-HTN allows insights into technical aspects of RDN and can thereby help in optimising future RDN trial design.