Cardiovascular medicine has undergone a momentous change over the past 2 decades with the development of percutaneous transcatheter interventions for structural heart disease. This field has the greatest growth in interventional cardiology for the foreseeable future.1–3 The number of patients with acquired valvular heart disease and adult congenital heart disease will only rise in the future as the population expands and ages. Multimodality imaging using cardiac CT, echocardiography, fluoroscopy, and fusion imaging are necessary in structural heart disease interventions. Transesophageal echocardiography (TEE), in particular, provides unique real-time 3D echocardiography of valvular heart disease and anatomic cardiac defects before, during, and after transcatheter therapies. This review will summarize the role of TEE in patients undergoing percutaneous mitral valve (MV) repair (MitraClip®), left atrial appendage (LAA) closure, transcatheter aortic valve replacement (TAVR), and atrial septal defect (ASD)/patent foramen ovale (PFO) closure.
Left Atrial Appendage Occlusion
Approximately 2.7 million–6.1 million people in the US have atrial fibrillation (AF).4 As the population ages, this number is expected to increase. AF increases the risk of ischemic stroke by five-fold and the strokes are typically more severe compared to non-AF-related strokes.5 Though oral anticoagulation (OAC) is the mainstay treatment for stroke prevention, up to 40 % of patients do not receive OAC, typically due to contraindications or bleeding risk.6 LAA occlusion devices are an alternative for patients with contraindications or prohibitive bleeding risk on OAC. The most common percutaneous occlusion devices include the WATCHMAN device (Boston Scientific Corp.), the AMPLATZER™ Cardiac Plug (St. Jude Medical), and the LARIAT® system (SentreHEART, Inc.). The LARIAT system may be used off-label for a similar indication, though the US Food and Drug Administration (FDA) has raised safety concerns in this setting.7 This review will focus on the role of TEE with the WATCHMAN device, since it is the only FDA-approved device for stroke prevention in non-valvular AF.
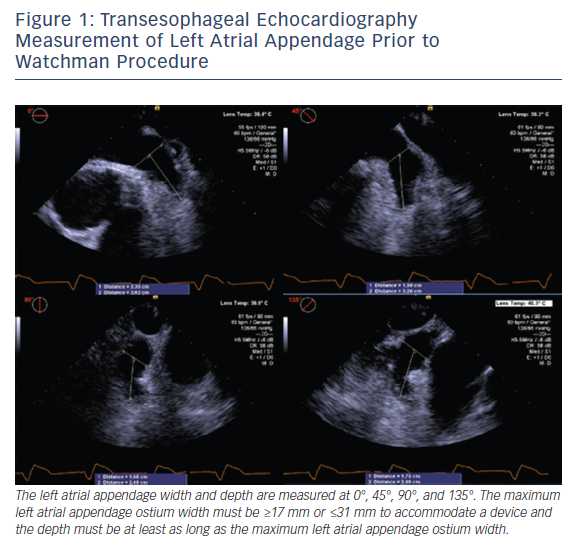
TEE assessment prior to WATCHMAN device placement includes: evaluation for LAA thrombus; LAA size, morphology, and the number and depth of the lobes; and assessment of other structural abnormalities. While meticulous evaluation for thrombus in the LAA from 0° to 180° is performed, the LAA ostium width and depth are measured at 0°, 45°, 90°, and 135°, see Figure 1. The ostium width is measured from the level of the left main artery or mitral annulus to a point 2 cm below the tip of the pulmonary vein ridge. The depth is measured from the ostium to the LAA apex. The maximum LAA ostium width must be ≥17 mm or ≤31 mm to accommodate a device and the depth must be at least as long as the maximum LAA ostium width.8–10 Real-time 3D TEE has emerged as a potentially faster and more accurate way to assess the size and morphology of the LAA. Small studies suggest the real-time 3D TEE assessment of LAA morphology is equivalent to MRI or CT, and superior to assessment using 2D TEE imaging.11–13 Three-dimensional assessment may also obviate the need to assess the LAA at multiple angles to obtain the maximum LAA ostium width and LAA depth.12,13 More widespread use of 3D TEE could potentially reduce cost and patients’ exposure to radiation/contrast; nonetheless, it is limited to centers with access to such technology.
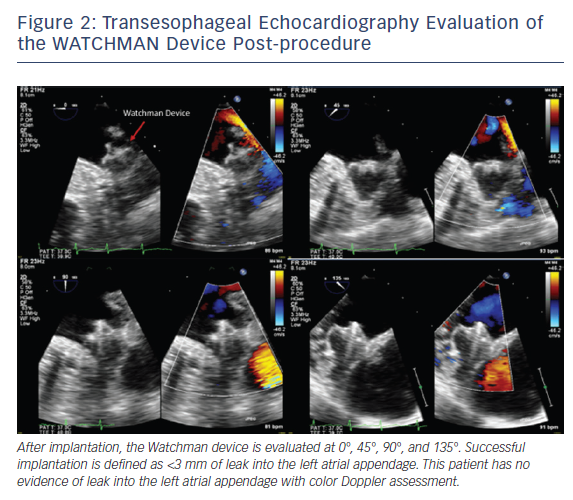
Intraprocedurally, TEE is used to initially guide transseptal puncture and to confirm optimal positioning of the delivery system. Optimal device placement is when the axis of the device aligns with the long axis of the LAA. Color flow evaluation over the device is needed prior to final deployment to assess for adequate LAA occlusion, defined as mild leak or less into the LAA with a jet diameter <3 mm, see Figure 2. Finally, at the end of the procedure, TEE is used to assess for changes in transmitral and pulmonary vein flow, along with potential complications including pericardial effusion, tamponade or device migration.8–10 Patients remain on aspirin and OAC for at least 30 days after device implantation, and a TEE is done to assess for thrombus and residual peri-device leak, with the same protocol at baseline. If residual flow into the LAA is >5 mm in diameter, then the patient should continue or restart anticoagulation.
Transcatheter Aortic Valve Replacement
Calcific aortic stenosis (AS) remains the most common form of valvular disease in developed countries, affecting approximately 2 % of people >65 years.14 Surgical aortic valve replacement is typically considered the treatment of choice for patients with symptomatic severe AS. However, many of these patients are considered inoperable due to significant surgical risk. The development of transcatheter aortic valve replacement (TAVR) gave high and intermediate risk patients an equally efficacious, but less invasive, option for valve replacement.15 TAVR requires multimodality imaging support. Specifically, echocardiography plays an essential role in the identification of patients with severe AS, sizing of the prosthesis, intraprocedural guidance, and the follow-up of prosthetic valve function.
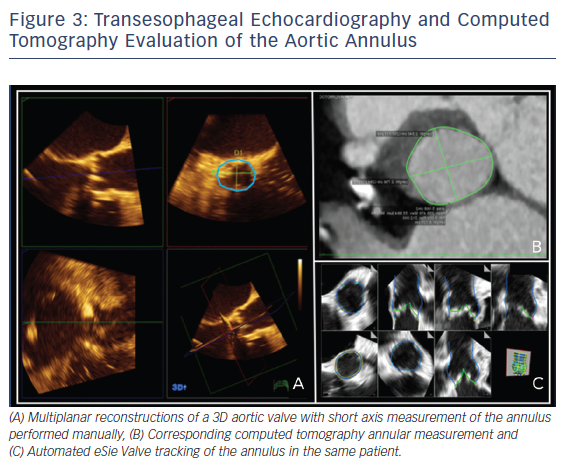
Two-dimensional and Doppler echocardiography remain the workhorses for the diagnosis of severe AS. TEE with Doppler is often less accurate due to difficulties with ultrasound beam alignment. However, TEE planimetry provides an alternative method for patients with suboptimal transthoracic echocardiography (TTE) evaluation. It correlates quite well with a continuity equation-derived valve area on TTE and with valve area derived from the Gorlin formula in the catheterization lab.16 Three-dimensional TEE, which obviates geometric assumptions of the elliptical aortic apparatus, may provide an even more accurate assessment of the left ventricular outflow tract/aortic annulus, aortic root, and aortic valve. Several studies have looked at annular sizing between 3D TEE and multidetector CT (MDCT) for TAVR size selection prior to implant, see Figure 3A and B. This question is important, since undersizing can result in device embolization or significant paravalvular leak, an independent predictor of mortality after TAVR.17 Similarly, oversizing can result in conduction abnormalities and aortic root rupture.17 Unfortunately, no clear answer exists. The majority of studies suggest that 3D TEE results in undersizing of the aortic annulus compared to MDCT, though with small absolute differences.18–22 In contrast, some small studies showed good correlation between 3D TEE and MDCT for annular sizing and for predicting paravalvular leak.23–26 At least some of this difference may be related to the lower spatial resolution of 3D TEE at the time, image dropout secondary to significant calcification at the annulus or aortic valve, and center experience.27 Notably, heavy aortic calcification limits 3D TEE evaluation of planimetry and annulus measurements due to image dropout. TEE with 3D and automated software, see Figure 3C, may ultimately provide pre-TAVR patients with ‘one-stop shopping’ for the evaluation of the entire aortic apparatus with excellent temporal and good spatial resolution, while avoiding contrast and radiation.
Concomitant TEE during the TAVR procedure aids in the placement of the valve, allows visualization of balloon aortic valvuloplasty, helps with sizing, identifies heavily calcified aortic annuli, identifies shallow coronary ostia origin, and provides rapid feedback to the interventionalist on complications. However, the routine use of intraprocedural TEE versus TTE proves controversial. The general trend is to use TEE-guided TAVR placement during cases necessitating general anesthesia, and those with transapical, transaortic or subclavian approaches for image quality reasons. A number of studies have shown that conscious sedation is becoming more prevalent with a transfemoral approach, and outcomes have been similar despite greater use of TTE over TEE.28–32 Regardless of the specific modality chosen, echocardiography plays an essential role in TAVR. The interventionalist and echocardiographer should localize the prosthesis below the aortic annulus and monitor its relationship to the MV and left ventricular outflow tract. The team must also look for the prosthesis to assume its predicted circular shape in the short axis view.9,33 Once implanted, TEE or TTE is used to confirm optimal anatomical position, aortic valve area >1.2 cm2, mean gradient <20 mmHg, peak velocity <3 m/sec, and absence of moderate or severe regurgitation.34
TTE is performed at 24 hours, 30 days, and then at least annually after the procedure. Evaluation must include TAVR function (aortic valve area, mean gradient, peak gradient and peak velocity), the presence of intravalvular/ paravalvuar regurgitation, right ventricular systolic pressure, and potential for other complications. These complications include left ventricular dysfunction related to coronary ostium occlusion, prosthesis migration/ embolization, MV damage, tamponade, and aortic root damage.35
Percutaneous Mitral Valve Repair
Degenerative MV disease is the most common cause of mitral regurgitation (MR).36 Surgical (MV) repair is the standard treatment to improve symptoms and prevent heart failure, with low mortality and high success rates at “mitral referral centers.” However, for patients who are high risk for surgery, palliative treatment with medications was the only choice until 2013, when MitraClip was FDA-approved for patients with symptomatic severe degenerative MR. The Efficacy of Vasopressin Antagonism in hEart failuRE: Outcome Study With Tolvaptan (EVEREST) trial in 2008 was the first randomized controlled trial comparing surgery versus percutaneous repair in high-risk patients. Most recently, a 5-year follow-up for durability was evaluated. While there were high rates of initial 3+ or 4+ MRs, this did not significantly increase over time. Additionally, there were no differences in long-term survival, left ventricular function, or dimensions. Although surgery has superior outcomes, there is evidence that a percutaneous alternative is safe, improves heart failure symptoms, and left ventricular dimensions.37
Identifying patients who meet the criteria for MitraClip starts with transthoracic echo imaging. Based on quantitative and qualitative parameters, patients must have moderate to severe or severe MR due to degenerative MV disease, left ventricular dimensions <6 cm, and left ventricular ejection fraction >20 %. TEE imaging is needed to confirm the severity of regurgitation, the jet origin (which ideally should be from the A2–P2 scallops), and the MV area (which should be >3 cm2). The prolapse or flail gap should be <10 mm and the width <15 mm. Patients with these echo criteria included in the EVEREST cohort were more likely to have a successful intervention without the need for a recurrent procedure. Patients with functional MR have recently been included in the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) trial. On TEE imaging, a coaptation length of ≥2 mm and a depth of <11 mm were needed for inclusion in the study.38–40
Relative echocardiographic contraindications include pathology at the body of the leaflet, severe calcifications at the site of grasping zone, small (<3.5 cm2) valve area and high gradients (>4 mmHg), small grasping zone <7 mm, Barlow’s disease with significant regurgitation of A1–P1 or A3–P3 scallops, and rheumatic disease.40 Though most of the assessment is 2D, 3D echocardiography should be used in the initial evaluation for MitraClip, since it may provide a better assessment of the etiology of regurgitation, dimensions of the MV, and an alternative to estimating severity.38–40
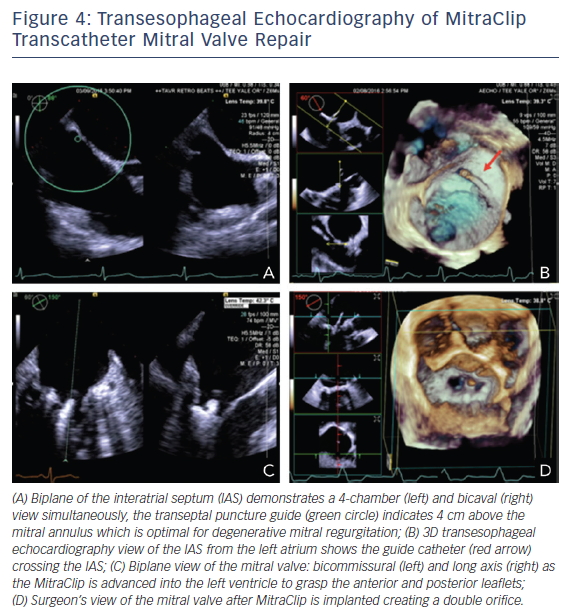
Three-dimensional TEE is an essential tool for the interventionalist in MitraClip procedures. Close collaboration between the primary operator and the echocardiographer is necessary to achieve successful placement. A complete 2D/3D TEE study prior to the procedure is paramount. Evaluation of the all pulmonary veins, in addition to all MR parameters, is invaluable to judge the success of the MitraClip procedure. TEE guidance begins with the transseptal puncture. Localization of the optimum position is posterior and superior in the septum with a height 3.5–4.0 cm from the MV annular plane depending on the MV pathology.41–43 Functional MR is due to tethering of the MV causing the mitral leaflets to be lower in the ventricle versus degenerative mitral leaflets, which are higher and are in the left atrium. Imaging planes for obtaining this optimal site include short axis with an angle of 30° at the base for anterior–posterior orientation, the bicaval at 90–120° for superior and inferior orientation, and a four-chamber at 0° to assist with height the annulus, see Figure 4A.42 The steerable catheter is introduced through the guide catheter, as seen on the 3D echocardiography in Figure 4B, and can be under continuous 2D echocardiography with biplane imaging as needed. The echocardiographer provides feedback on the proximity of the tip of this catheter and the wall of the left atrium. The MitraClip is directed to the left atrial appendage and then flexed downwards to the MV while using 3D imaging. A surgical view of the MV from a left atrial orientation allows positioning of the MitraClip perpendicular to the coaptation line and directly over the origin of MR, see Figure 4D.42 Occasionally, to confirm the position of the clip, a surgical view of the MV with decreased 3D enables the opened clip orientation to be viewed. The device is advanced into the left ventricle using biplane imaging, preferably using a bicommissural view and long-axis view on the opposite plane, see Figure 4C. The clip is positioned in the long-axis view to grab the anterior and posterior leaflets using 2D echocardiography. Successful placement of the clip is seen if the severity of MR decreases to <2+.42 Evaluation of MR should include regurgitant volume by continuity equation, vena contracta when possible, and pulmonary vein evaluation. The flow convergence method is challenging, since it may overestimate MR when assessing multiple jets and underestimate MR in eccentric jets.42 Equally important is the assessment of MV stenosis, particularly with multiple MitraClips prior to device release. A complete post-device evaluation should determine the presence of pericardial effusion, ventricular or leaflet perforation, and residual iatrogenic ASDs.42 Patients with shunts post procedure do worse clinically. The shunt assessment should be done with 3D TEE, since it has proven to be more accurate in predicting size compared to 2D TEE, which underestimates diameter and area.44,45
The EVEREST cohort had strict and specific echocardiographic variables that did not reflect the real-life situations of patients. A 12-month followup study compared patients who met strict EVEREST criteria to patients representing a real-life cohort.46 Acute and 30-day outcomes were similar. At 1 year, reduction in MR and improvement in left ventricular dimensions were also comparable. This study argues that the MitraClip should be expanded to those patients with less ideal echocardiographic features.46 More recently, a long-term outcomes study compared the patients that fulfilled the strict inclusion criteria to those who did not. Regardless of EVEREST criteria, the authors found a 95 % primary success rate and a significant reduction in MR severity. However, those who did not meet the strict criteria were more likely to require an additional intervention due to recurrent MR.47
Atrial Septal Defect/Patent Foramen Ovale Closure
Catheter intervention with the assistance of echocardiography has created a suitable alternative for the closure of uncomplicated secundum ASD and PFO. The indications for ASD closure include right ventricular and atrial enlargement, evidence of paradoxical embolism, or documented orthodeoxia-platypnea, and may be considered when a significant shunt is present.48 Currently, there are no approved indications for PFO closure other than documented orthodeoxia-platypnea, although catheter intervention can be used off-label for paradoxical embolization.49
TTE with agitated saline is usually the initial step in the detection of atrial shunts and in assessing the hemodynamic consequences.50 A TEE should be considered when the TTE is negative but high clinical suspicion remains, or to confirm an ASD or PFO, evaluate for pulmonary shunt, and define anatomy.50 There are defined imaging planes on TEE that are used to assess the size and rims of an ASD. TEE should begin with an entire sweep of the atrial septum at 0° (four-chamber) followed by 90° (bicaval view) with and without color Doppler. At 0°, an investigation of the interatrial septum (IAS) starts from superior vena cava down to the inferior vena cava, and at 90° an entire sweep from right-to-left should be viewed. At 30° the anterior portion of the IAS adjacent to the aorta and the posterior rim is assessed.50,51
There are a number of anatomical features that are required to confirm catheter closure suitability and device choice. Defects >38 mm and those with a deficient rim <5 mm may require surgery, though deficient rims anterior adjacent to the aorta are less problematic.52 Transcatheter repair of ASDs that are in proximity to the mitral and tricuspid valve and deficient with rims adjacent to the superior vena cava and inferior vena cava are typically avoided. There are several types of device available, and the presence of certain anatomical features will sway the use of one device over another. For example, the GORE® HELEX® device (W. L. Gore & Associates) can be considered in place of the widely used AMPLATZER™ Septal Occluder (St. Jude Medical) when there is a deficient rim, since its softer disks may lead to less erosion.52 Real-time 3D TEE helps to define the shape, size and rim of the ASD, since it provides an enface view. This image can then be rotated to view the septum from the right and left atrium, enabling us to appreciate the dynamic geometry in diastole and systole. The pre-assessment for a PFO should include size of the left and right atrial opening, total length of the PFO tunnel, the presence of aneurysm, and the location and extent of the Eustachian ridge and valve. The pre-assessment for ASD closure includes thickness of the secondary septum, rim size, and defect size.9,50,51
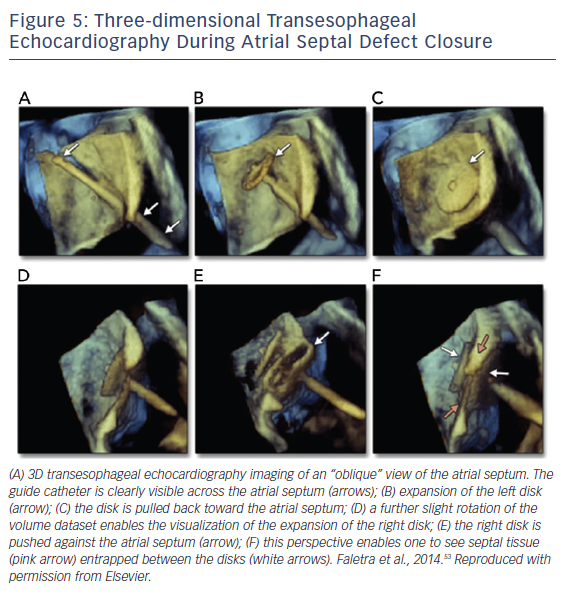
Once appropriate patients and devices are selected, 3D TEE can assist in the septal puncture from the oblique and lateral views, and in the case of ASDs it has been shown to provide better definition of septal anatomy and visualization of catheters. From the oblique and lateral views, the guide catheter can be seen clearly crossing the IAS. While in this view, rotating from right to left creates a view of the catheter in the left atrium, allowing for disk expansion. A lateral perspective allows visualization of the right disk expansion, resulting in the IAS being sandwiched by two expanded disks, see Figure 5.53 In addition to 3D visualization, biplane imaging aids in the placement of the device, ensuring alignment with the IAS. A successful closure is defined by complete closure with residual shunt <1–2 mm.52 With regard to long-term follow up, TTE is sufficient to assess for migration, residuals shunts, right atrial and right ventricular size, and pulmonary artery pressure, if available.9
Conclusion
The trend towards developing less invasive procedures for structural heart disease has led to an increased need for echocardiography support in the diagnosis of disease, sizing of devices, procedural guidance, and monitoring to determine the success or failure of procedures. There is a high demand for improved spatial and temporal resolution via 2D and 3D imaging to meet these expectations. Evolving technology is expanding the role and improving the accuracy of 2D and 3D TEE and TTE in the rapidly-growing fields of structural cardiology and interventional echocardiography.