Newer generation polymeric metallic drug-eluting stents (DES) have shown improved efficacy and safety compared with bare-metal stents and first-generation DES, improving patient outcomes after percutaneous coronary intervention (PCI) and facilitating the treatment of more complex coronary disease.1 However, clinical outcomes in certain lesion and patient subsets remain suboptimal. Procedural and/or technological refinements may improve success rates in such scenarios. Patients with diabetes represent one such complex subgroup as they continue to have worse outcomes following PCI compared with patients without diabetes.2 The latest DES technological enhancements may have an important impact in improving the outcomes of these patients.
The Cre8™ EVO (Alvimedica) represents novel DES technology that features laser-cut reservoirs on the abluminal surface of the stent that support the controlled polymer-free elution of Amphilimus™. In the current review, we discuss coronary revascularisation in patients with diabetes, with a focus on the latest efficacy and safety data from studies evaluating the Amphilimus™ polymer-free DES and its latest iteration, the Cre8™ EVO stent, in patients with diabetes.
Clinical Efficacy of DES in Patients with Diabetes
Diabetes has become a global health emergency. At present, an estimated 415 million adults have diabetes worldwide.3 By 2040, this is projected to rise to 642 million.3 In addition, it is estimated that there are 192.8 million people worldwide with undiagnosed diabetes.3 More than 25 % of patients referred for coronary revascularisation procedures have diabetes. Such patients have a higher risk of complications compared with patients without diabetes, and their long-term prognosis is worse in terms of restenosis, stent thrombosis, myocardial infarction (MI) and death.4–8 Clinical outcomes in diabetic patients treated with DES versus bare-metal stents, as well as the comparative performance of several DES, have been assessed in randomised trials and large observational registries.9 The 300-patient randomised Comparison of Everolimus-eluting Stent versus Sirolimus-eluting Stent Implantation for de novo Coronary Artery Disease in Patients with Diabetes Mellitus (ESSENCE-DIABETES) trial succeeded in showing non-inferiority of everolimus-eluting stents (EESs) compared to first-generation sirolimus-eluting stents with respect to angiographic late lumen loss (LLL) at 8 months with no significant difference in clinical outcomes at 1 year, although the trial was not powered to show a statistical difference with respect to the latter.10 A pooled analysis of 6,780 patients treated with second-generation EES versus first generation paclitaxel-eluting stents enrolled in the Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) II, SPIRIT III and SPIRIT IV and the Second-Generation Everolimus-Eluting and Paclitaxel-Eluting Stents in Real-Life Practice (COMPARE) randomised trials showed that despite improved safety and efficacy of EES in non-diabetic patients at 2 years, there was no difference between the devices with respect to outcomes in diabetic patients (n=1,869).11 Furthermore, different second-generation DES devices – utilising permanent or bioresorbable polymers – have not demonstrated differential efficacy in patients with diabetes.12,13 In scanning electron microscopy studies, cracks and inhomogeneous distribution of coating have been observed on all DES types assessed.14,15 Such occurrences can promote platelet aggregation, stent thrombosis and, in individuals with diabetes, trigger an inflammatory response within the vessel wall, potentially accelerating progression of atherosclerosis and risk of restenosis.16
Revascularisation in Diabetic Patients with Multivessel Coronary Artery Disease
European guidelines for clinical practice recommend coronary artery bypass graft (CABG) surgery in preference to PCI in diabetic patients with multivessel disease, with PCI considered a treatment alternative in patients with a low SYNTAX score (≤22).17 However, randomised trials comparing PCI with CABG in patients with diabetes are somewhat outdated. The largest trial to compare PCI with CABG for the treatment of multivessel coronary artery disease in diabetic patients was the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial, which found CABG to be superior to PCI with respect to the primary endpoint, the combined incidence of death, non-fatal MI or stroke. This was driven by a reduction in both non-fatal MI and death in the CABG group, albeit with an almost two-fold higher incidence of stroke.18 However, the trial is limited by the use of first-generation DES in 94 % of patients in the PCI group. Moreover, of 33,000 patients screened, only 1,900 (5.7 %) were enrolled, only 2.5 % of enrolled patients had a left ventricular ejection fraction <40 %, and only 35.5 % had a SYNTAX score ≤22 – all factors limiting the external validity of results.
Other randomised trials comparing PCI and CABG were underpowered with respect to their primary outcome measures. Both the Coronary Artery Revascularization in Diabetes (CARDia) study19 and the Veterans Affairs Coronary Artery Revascularization in Diabetes Study (VA CARDS) were terminated early due to slow enrolment.20 The CARDia trial enrolled 510 of the 600 patients planned and failed to show non-inferiority of PCI versus CABG with respect to the combined incidence of death, MI or stroke. VA-CARDS randomised only 207 (3 %) of 6,678 patients screened, representing only one-quarter of the planned sample size. Both trials were also limited by the use of first-generation DES as well as bare-metal stents in the CARDia study. Finally, a subgroup analysis of patients with diabetes enrolled in the Synergy between PCI with TAXUS and CABG (SYNTAX) study (n=452)21 found no significant difference in the combined incidence of all-cause death, MI or stroke between the two groups, although the trial was not designed to show such a difference in subgroups. Despite the fact that patients with diabetes fared worse than patients without diabetes in the SYNTAX trial, the presence of diabetes was not found to be independently associated with increased risk of major adverse cardiac events in multivariable analysis.
It is clear that only one randomised study comparing PCI and CABG in patients with diabetes was adequately powered to show a difference between treatment groups with respect to its primary endpoint, and that all trials are limited by the use of predominantly first-generation DES. Irrespective of the revascularisation strategy, patients with diabetes are at a higher risk of progression of de novo native coronary artery disease compared with patients without diabetes. Furthermore, while angiographic patency of the internal mammary artery has been demonstrated to be >90 % at follow-up of ≥10 years, venous bypass grafts tend to degenerate much earlier and existing randomised data do not address the comparative efficacy of PCI versus CABG beyond 5 years. An unmet need remains for a contemporary comparison of CABG surgery versus PCI in patients with diabetes using a newer-generation DES – preferably a DES specifically designed to treat patients with diabetes – and highly potent antiplatelet therapies with superior antithrombotic efficacy over clopidogrel with prospective long-term follow-up.22
Design Features of the Cre8™ Stent
The mammalian targets of rapamycin (mTOR) inhibitors (-limus drugs) are less active in people with diabetes compared to those without the condition. One reason for this is the direct resistance of vascular smooth muscle cells to mTOR inhibition in people with diabetes.23,24 The mTOR receptor is involved in the restenotic response cascade caused by vascular injury through the degradation of p27kip1 – a cyclin-dependent kinase inhibitor. Dysregulation of mTOR and p27kip1, coupled with an increase in the activity of the extracellular signal response kinase 1/2, has been found to promote intimal hyperplasia in the setting of diabetes mellitus.23 Dose–response curves have shown that a 10-fold higher concentration of mTOR inhibitor is required in a diabetic cell to achieve a similar level of inhibition to a non-diabetic cell.24
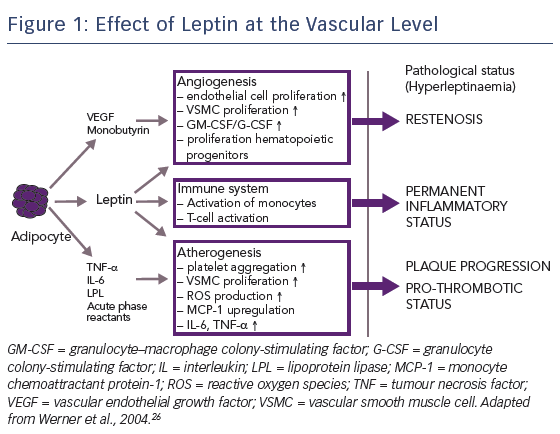
Another factor contributing to the relative lack of efficacy of mTOR inhibitors in patients with diabetes is the impact of hormones. Being overweight is a strong risk factor for diabetes and cardiovascular disease; >90 % of patients with type 2 diabetes are overweight or obese.26 Human obesity is associated with elevated levels of leptin, a hormone secreted by adipocytes and perivascular tissue, particularly in overweight patients. Leptin has a number of specific vascular effects: first, it promotes angiogenesis, which contributes to restenosis; second, it activates the immune system, resulting in a permanent inflammatory status; and third, it accelerates atherogenesis, promoting plaque progression, see Figure 1.27,28 A nine-fold increase in the dose of sirolimus has been shown to be required to inhibit leptin-induced intimal hyperplasia.29 Therefore, in order to improve PCI efficacy in patients with diabetes, the concentration of drug level attained in diabetic cells needs to be increased.
Other features of the Cre8™ DES may optimise clinical efficacy in patients with diabetes. First, the Amphilimus™ formulation is a proprietary technology in which sirolimus and a fatty acid are combined and eluted together to achieve enhanced drug bioavailability, more homogeneous drug distribution and greater drug stability.30 Sirolimus is a highly potent immunosuppressant drug with anti-proliferative and anti-microbial activities as well as anti-inflammatory properties. The Amphilimus™ formulation of Cre8™ makes use of the key role of fatty acids in cellular metabolism in patients with diabetes. While in the non-diabetic cell, fatty acid metabolism is responsible for 70 % of adenosine triphosphate generation (the remainder being produced by glucose oxidation), in the diabetic cell fatty acid oxidation is responsible for 100 % of adenosine triphosphate production due to membrane protein overexpression, which results in the higher binding and translocation of fatty acids.31
Another key feature of the Cre8™ DES is abluminal reservoir technology: a proprietary polymer-free drug-release system consisting of reservoirs on the stent’s abluminal surface which direct drug release exclusively towards the vessel wall. This has proven the only polymer-free technology capable of providing the same sustained elution kinetics as the most effective polymeric DES, allowing peak drug-tissue concentration during the first days after implantation, 50 % drug elution within approximately 18 days, 65–70 % elution within 30 days and complete drug elution within 90 days.32 Unlike polymers, which determine the speed of release of substances according to their size, abluminal reservoirs allow a mix of substances to be simultaneously eluted for maximised synergetic effect; the kinetic release being determined by the reservoir shape.30
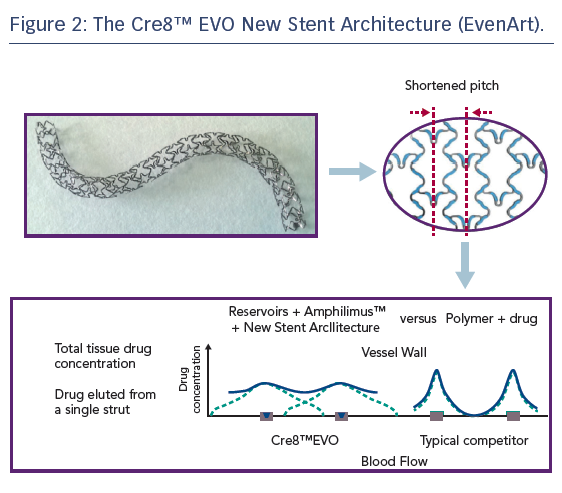
Refinement of the Cre8™ stent architecture aims to further improve homogeneity of drug distribution within the vessel wall, particularly in the case of the complex coronary anatomies and pathologies typically encountered in patients with diabetes. The new stent architecture (EvenArt) features a shortened pitch with reduced crown width and a different link number and pattern compared with the previous design, the combination of which results in an enhanced elution profile, see Figure 2. The reservoirs are closer together, ensuring more homogeneous drug deliverability.33 The new design also results in greater conformability,33 which is particularly important in individuals with diabetes, who tend to present with smaller vessels, a higher incidence of multivessel disease, more lesions in proximal locations (including the left main coronary artery), impaired circulation and increased coronary artery calcification.34 Additional features of the stent include thin struts (70/80 μm) consisting of cobalt–chromium; a Bio Inducer Surface (consisting of pure carbon); no stent shortening upon expansion; and two platinum markers at the stent ends. Delivery system features include a balanced and hydrophilic coated shaft, short balloon tapers (or shoulders) – which aid device deliverability and minimise trauma to the adjacent vessel, while reducing the risk of stent displacement on the balloon during dilatation – and a balloon-rated burst pressure of 18 atm across the entire product range, which includes diameters from 2.25 mm to 4.5 mm and nominal lengths of 9–46 mm.33
Clinical Evidence for use of the Cre8™ stent in Patients with Diabetes
Randomised Studies
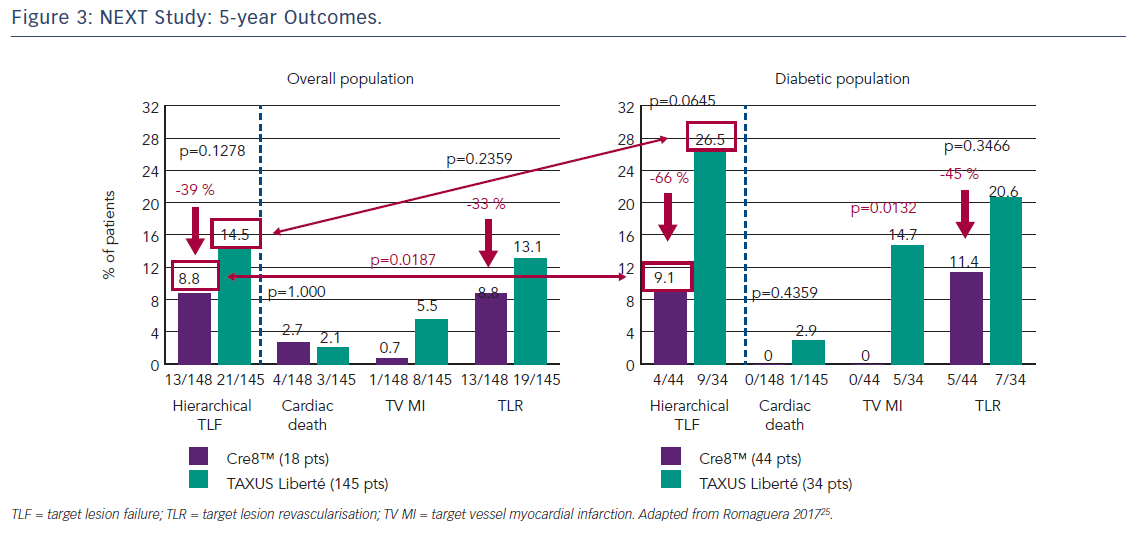
The first clinical study to demonstrate the clinical efficacy of the Cre8™ DES was the International Randomized Comparison Between DES Limus Carbostent and TAXUS Drug-eluting Stents in the Treatment of De Novo Coronary Lesions (NEXT) trial, which allocated patients with ischaemic myocardial symptoms related to de novo lesions in native coronary arteries (n=323) to treatment with the Cre8™ DES or a paclitaxel-eluting stent (TAXUS™ Liberté®, Boston Scientific).35 The primary endpoint was 6-month angiographic in-stent LLL. At 6 months, the Cre8™ group had reduced LLL by 60 % compared with the TAXUS group (p<0.0001). This finding was even more pronounced in the subgroup of patients with diabetes (n=82), where a 72 % reduction in LLL was observed (p<0.0001). At 5 years, patients in the Cre8™ group showed a trend towards a 39 % reduction in cumulative target lesion failure (TLF, the combined incidence of cardiac death, target vessel MI and all target lesion revascularisation [TLR]) compared with the TAXUS groups (8.8 % versus 14.5 %, respectively; p=0.13) and a reduction of 66 % in the diabetic subgroup with a trend toward statistical significance (9.1 % versus 26.5 %, respectively; p=0.06), see Figure 3.25 These trends were driven by a significant reduction in target vessel MI in the Cre8™ group in both the overall and diabetic populations. It is notable that among patients treated with the Cre8™ stent, the rate of TLF in the diabetic subgroup was similar to that of the overall study population.36
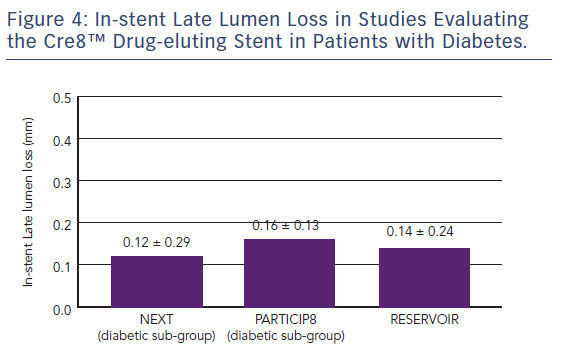
The Randomized Comparison of Reservoir-based Polymer-free Amphilimus™-eluting Stents versus Everolimus-eluting Stents in Patients with Diabetes Mellitus (RESERVOIR) clinical trial enrolled 112 patients with diabetes on glucose-lowering therapy with a single de novo lesion in a maximum of two coronary arteries.37 Participants were randomised to treatment with the Cre8™ stent (n=56) or a XIENCE™ (Abbott) EES (n=56). The primary endpoint was mean neointimal hyperplasia volume obstruction as measured by optical coherence tomography after 9 months, and was numerically lower in the Cre8™ group compared with the XIENCE group (11.97 ± 5.94 % versus 16.11 ± 18.18 %; p=0.0003 for noninferiority; p=0.22 for superiority). Prespecified subgroup analyses showed a consistent treatment effect in favour of the Cre8™ arm across all subgroups. Importantly, this difference was statistically significant in the subgroup with suboptimal metabolic control (Hb1Ac greater than the median value; p=0.02). In terms of secondary endpoints, angiographic LLL in the Cre8™ group was 0.14 ± 0.24 compared with 0.24 ± 0.57 in the XIENCE group, with a striking difference in standard deviations that highlights the consistent performance of the Cre8™ DES. There was also a trend towards a larger minimum lumen diameter in the Cre8™ versus the control group in-stent (2.38 ± 0.44 versus 2.19 ± 0.59; p=0.07) and in segment (2.09 ± 0.59 versus 1.84 ± 0.61; p=0.02). Notably, the degree of LLL observed in RESERVOIR was consistent with that already seen in the NEXT trial and the Prove Abluminal Reservoir Technology Clinical benefit in all comers patients (pARTicip8) observational study, see Figure 4. There were no differences between the Cre8™ or XIENCE groups with respect to any clinical endpoint, including cardiac death (1.8 % versus 0 %, respectively; p=1.0), myocardial infarction (0 % versus 1.8 %, respectively; p=1.0), definite or probable stent thrombosis (1.7 % versus 1.7 %, respectively; p=1.0) or repeat revascularisation (TLR 5.2 % versus 8.6 %, respectively; p=0.46). However, the study was underpowered to detect such differences between treatment groups.
Non-randomised Studies
The pARTicip8 study recruited 1,186 patients with de novo lesions in native coronary arteries and symptoms of myocardial ischaemia at 30 European sites. The study aimed to evaluate the safety and efficacy of the Cre8™ stent in a broadly inclusive real-world population, with a specific focus on diabetic subgroups. The primary endpoint was a composite of cardiac death, target vessel MI and clinically-indicated TLR at 6-month follow-up. Of the patients enrolled, 308 (25.8 %) had diabetes. At 1 year, the incidence of the primary endpoint was 2.8 % in the overall population and 4.7 % in the diabetic subgroup.36
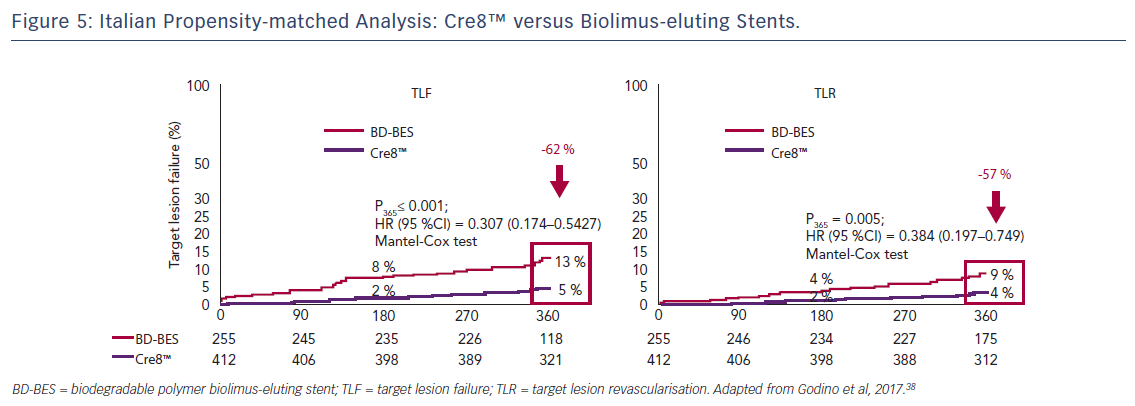
In a propensity-score-matched analysis, patients from the Amphilimus™ iTalian mUlicenTre rEgistry (ASTUTE) database were matched with patients from the Italian Nobori Stent ProspectIve REgistry (INSPIRE-1) who were treated with biolimus-eluting stents during the same period. In the diabetic subgroup, a 62 % reduction in TLF (5 % versus 13 %; p<0.001) and a 57 % reduction in TLR (4 % versus 9 %; p=0.005) were observed in the Cre8™ versus the biolimus-eluting stents groups, see Figure 5. Stent type was found to be an independent predictor of TLF, with an odds ratio of 2.76 (95 % CI 1.36–5.56).38
Future Randomised Studies
While the growing body of randomised and observational data supports the efficacy of the Cre8™ DES, studies to date have not been powered to demonstrate superiority over comparator DES. Against this background, a large randomised study – the Second-generation drUg-elutinG stents in diAbetes: a Randomised Trial (SUGAR Trial) – is planned. The trial aims to randomise 1,164 “all-comer” patients undergoing PCI at 29 centres in Spain to treatment with the amphilimus-eluting stent or a zotarolimus-eluting stent in a 1:1 ratio. The primary endpoint, TLF – defined as the composite of cardiac death, target vessel MI and TLR – will be assessed at 12 months for non-inferiority and at 24 months for superiority.
A second randomised trial, the Clinical benefit in “all comer” patients with DIABetes to prove Cre8™ EVO (Diab8) study,39 aims to recruit 3,040 all-comer patients with diabetes undergoing PCI at 54 international sites. Patients will be randomised in a 1:1 treatment allocation to the Cre8™ EVO stent or an EES. The primary endpoint is TLR at 12 months. There will be a sequential analysis for non-inferiority and then for superiority. Secondary endpoints include cardiac death and target vessel MI at 12 months and TLR (superiority) at 24 months.
In summary, the Cre8™ DES has shown promising results in the treatment of patients with diabetes in both subgroup analyses of randomised trials and observational studies. The large-scale SUGAR Trial and Diab8 study aim to provide randomised evidence to support the efficacy and safety of the Cre8™ and Cre8™ EVO stents in patients with diabetes.
Summary and Concluding Remarks
The rising prevalence of diabetes worldwide has made effective treatments for people with diabetes an urgent priority. However, current revascularisation strategies remain suboptimal in this subset of patients. Patients with diabetes have worse outcomes compared with the general patient population following revascularisation, and second-generation DES have failed to significantly impact on outcomes in such patients. The Cre8™ DES utilises abluminal reservoirs that slowly elute the Amphilimus™ formulation to overcome -limus resistance and the proliferative effect of hormones in diabetic cells, and thus increase cellular uptake of sirolimus. The new stent architecture of the Cre8™ EVO stent – EvenArt – is designed to enhance the elution profile in challenging anatomies. Finally, a growing body of randomised and observational data suggests favourable comparative performance of the Cre8™ DES with other DES in patients with diabetes. The results of large-scale randomised trials specifically comparing the Cre8™ or Cre8™ EVO DES with current-generation DES in diabetic patients are eagerly awaited.