In the United States, atrial fibrillation (AF) is the most common sustained cardiac arrhythmia affecting approximately six million patients and contributing to a greatly increased risk of stroke, heart failure (HF) and overall morbidity and mortality.1,2 The prevalence of AF is increasing as the average age of the population increases.3,4
Currently available therapies for AF are suboptimal. Therapeutic options include sinus rhythm restoration and/or ventricular rate control. Both are achieved by pharmacological or ablation therapy. Efficacy is limited, and the risk of adverse effects to therapy is increased in patients with long-standing persistent AF and comorbidities that commonly accompany AF, such as HF or lung disease.5 Antiarrhythmic drug use has potential risks such as pro-arrhythmia and non-cardiovascular toxicities.6–8 AF ablation therapy has become increasingly popular, but efficacy is limited, with high recurrence rates requiring repeated procedures.9,10 Severe complications (including mortality of 0.1 % in a recent survey) remain a persistent problem for AF ablation.11 Limitations in currently available therapies dictate a need for novel and more effective therapies.
Cardiovascular gene therapy has the potential to expand treatment options for AF. Recent improvements in gene transfer vectors and delivery methods and a deeper understanding of molecular mechanisms of AF increase the probability that gene therapy will successfully translate to a clinically viable therapy over the next few years. In this review, we will analyse the available vectors and delivery methods for myocardial gene therapy and evaluate the current stateof- the-art for AF gene therapy.
General Principles of Myocardial Gene Transfer
Gene therapy is the delivery of functional genes into a target cell or tissue for the treatment or prevention of disease. Three major components for successful gene therapy are the selection of a gene transfer vector, a delivery method and a therapeutic gene target.
Vectors
Generally, vectors for gene delivery fall into one of two different kinds: viral or nonviral vectors. Nonviral vectors are DNA plasmids, alone or in combination with adjuncts that improve delivery.12,13 The advantage of naked DNA (plasmid with no complexing agent) is its inherent simplicity. DNA in the form of a plasmid is relatively easy to grow, purify and use, and it has documented acceptance by regulatory bodies. The major problem with nonviral vectors is inadequate transfection efficiency.14 Most studies that have assessed in vivo gene delivery with nonviral vectors have shown very limited uptake by target cells, with at most a few percent of target cells expressing the transgene. Complexing agents (lipid, carbohydrate or protein coatings) increase delivery to a limited degree, but these agents also increase toxicity. Some physical methods (electroporation, ultrasound disruption of microbubbles) have been developed to enhance gene transfer, but the efficiency in vivo still remains low.14–18
Viral vectors are more frequently used for cardiovascular diseases due to their superior efficiency in cellular uptake and gene expression compared with nonviral vectors. The viral vector genome is altered by removing genes essential for virus replication so that viral vectors can only grow under special, supported circumstances and not in the target tissue (except conditionally replicating adenoviruses used in cancer gene therapy that are not relevant to this AF discussion). The three most commonly used viruses for cardiovascular applications are adenoviruses (Ad), adeno-associated viruses (AAV) and lentiviruses.
Ad are double-stranded DNA viruses with a 35 kb genome. First generation Ad have deletions of a limited number of viral genes, preventing virus replication and creating space for gene insertions up to 10 kb. Helper-dependent Ad vectors have the entire viral genome removed. Ad vectors have been widely used in the myocardial gene transfer literature, mainly due to their high transduction efficiency in cardiac myocytes and capability of generating peak expression over a short time period. The main disadvantage of Ad is the ability to elicit a profound immune response from the recipient. This immune response limits duration of gene expression to 2–4 weeks in vivo, depending on the target organ and transgene.19 Immune responses to Ad can cause organ damage and systemic inflammatory responses.20 Ad vectors have been used in a number of myocardial gene therapy clinical trials that have not yet shown efficacy (due in large part to limitations in delivery) but that also have not shown any detectable toxicity.21–23
AAV is a small virus with a linear 5 kb single-stranded DNA genome containing two genes: rep and cap.24 Recombinant AAVs have many desirable properties for cardiac gene transfer, including long-term (potentially permanent) gene expression and a relatively limited immune or inflammatory response by the host organism.25 Among the reported AAV serotypes, AAV1, 6, 8 and 9 are most commonly used for cardiac gene therapy.26,27 AAV gene therapy was initially tested on rodents, where dense cardiac delivery was demonstrated. It is not nearly as effective in large mammals as it is in mice, but it appears sufficient to alter the phenotype in large mammalian models of disease.28,29 The principal advantage of AAV is the possibility of permanent gene expression. Cardiac studies have been limited, but gene expression in skeletal muscle persisted over one year after injection in a haemophilia clinical trial.30 Preclinical large mammalian models with various targets, delivery techniques and transgenes have reported stable expression persisting for several years after gene transfer.31–33 The principal disadvantage of AAV vectors is the limited insert size, preventing use with some ion channels or other large genes. Recent cardiac studies have shown efficacy of AAV gene therapy in large mammalian models and in a human HF clinical trial.28,34
Lentiviruses are human immunodeficiency virus-based members of the retrovirus family. They are enveloped RNA viruses capable of packaging approximately 10 kb of genetic information. Unlike other retroviruses, lentivirus can transduce non-dividing cells, including cardiomyocytes. Transfection efficiency for lentivirus vectors is similar to AAV.30 Longterm stable gene expression is another similarity between lentiviruses and AAV, although by a different mechanism. Lentiviruses actually integrate into the host genome allowing permanent expression. A potential risk for lentiviruses is the possibility of mutagenesis related to the insertion site. Another significant limitation is the inability of current technology to concentrate them to levels necessary for intracoronary delivery. Lentivirus vectors have been used for intramyocardial injection in large mammalian models of cardiac disease. The feasibility of using lentivirus vectors in situations needing widespread cardiac delivery has not yet been verified and no clinical trials using lentivirus vectors for cardiac disease have been attempted to date.
Gene Delivery Methods
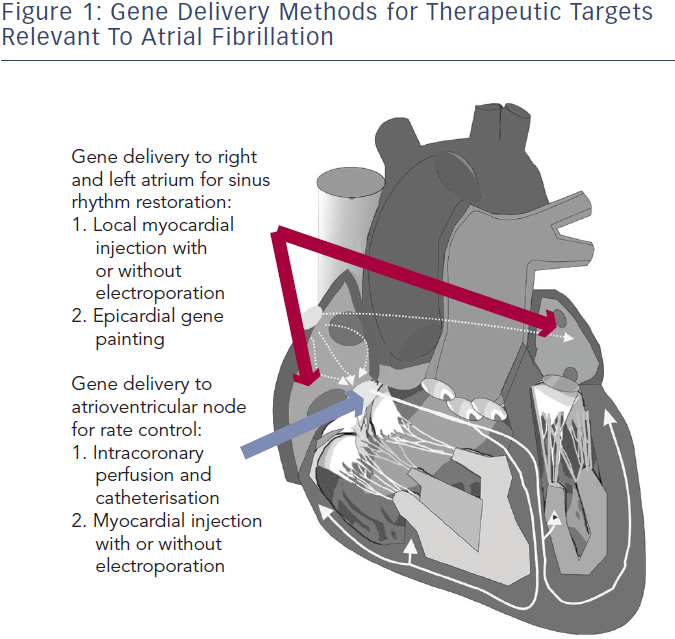
Several gene delivery strategies have been developed and verified for the large mammalian heart (see Figure 1). For AF therapy, the areas of therapeutic interest are primarily broad atrial delivery for sinus rhythm restoration and atrioventricular (AV) nodal delivery for rate control. Methods to deliver a gene transfer vector to these regions reported in preclinical AF models include direct myocardial injection followed by electroporation, epicardial gene painting and intracoronary catheterisation.31–34 Some methods such as intravenous injection and tail vein injection have been reported in mice, but these have not shown viability in large mammals.35,36
Direct myocardial injection is one of the simplest methods for effective cardiac gene delivery. Both naked DNA plasmids and viral vectors have been tested with this method.37,38 Intramyocardial injection leads to focused, high-density gene expression, but gene delivery is limited to the tissue volume within a few millimetres of the needle track.39,40 Thus, multiple injection sites would be required to achieve sufficient gene delivery in the large mammalian heart, which increases both the risk of adverse events during the procedure and the probability of heterogeneous gene expression. Injection-related tissue damage also has a risk of triggering an acute inflammatory response.41,42
In order to enhance efficiency, electroporation has been used immediately after plasmid or virus injection. Since its initial trial on skeletal muscle, electroporation-mediated nonviral gene therapy has improved efficiency in both small and large mammalian hearts in vivo.15,43–45 Thomas et al. and Aistrup et al. have demonstrated viability of direct myocardial injection with epicardial electroporation for gene delivery to the atria.34,46-48 In their studies, epicardial electroporation increased the efficiency from ~10 % to ~50 % in both atria. The drawbacks of this hybrid injection/electroporation approach include possible fibrillation of the heart if the pulse is not synchronised and challenges to coordinate the injection with the placement of the electrodes for routine clinical use.15 Targeting for direct injection has been reported with various imaging modalities, such as magnetic resonance imaging (MRI), cardiac CARTO-NOGA mapping (Biosense Webster Inc, CA, US) and ultrasound. These have not yet been reported in AF studies, but they could potentially be adapted for atrial or AV nodal gene delivery.49–52
To date, the atrial epicardial gene painting method is the only reported widespread atrial gene transfer method that achieves dense, transmural, homogenous atrial expression without affecting ventricular cardiomyocytes.33 Gene painting involves applying a mixture of poloxamer gel, dilute trypsin and gene transfer vector to the atrial epicardial surface. Poloxamer gel is used to increase virus contact time with the atria and trypsin increases virus penetration. The main technical difficulty to translating painting is the current need for open access to the atrial epicardial surface. In clinical settings, this delivery method could potentially be performed during open cardiac surgery or cardiac allografting.53 Modifications to create a minimally invasive method for this technique have not yet been reported.
Intracoronary perfusion is an attractive method for either whole heart or targeted AV nodal gene delivery, but it is not effective for isolated atrial gene delivery due to the lack of sufficient atrial vasculature.54 The advantages of this approach include minimal invasiveness and delivery of vectors using clinically standard equipment. The main disadvantage of delivery by intracoronary perfusion is the limited efficacy of whole heart delivery in spite of several reports that have outlined various parameters that can optimise gene transfer.55–59 To date, the best available method requires nitroglycerin, adenosine, vascular endothelial growth factor and low calcium administration to increase transvascular access, and simultaneous coronary arterial and venous delivery to increase target area. Sasano et al. used these methods with Ad vectors and achieved 80 % transfer to the anterior-septal left ventricle.32 A modification to achieve dense, whole heart delivery has not yet been reported.
Gene delivery methods are arguably the principal limitation to clinical translation of AF gene therapy. Myocardial injection and intracoronary infusion have been used in angiogenesis and heart failure clinical trials.60,61 As noted above, these methods would be limited for widespread, specific atrial delivery in the clinical setting. The epicardial painting method has potential for clinical application during cardiac surgery, and the development of a minimally invasive version could potentially lead to use in non-surgical AF therapy.
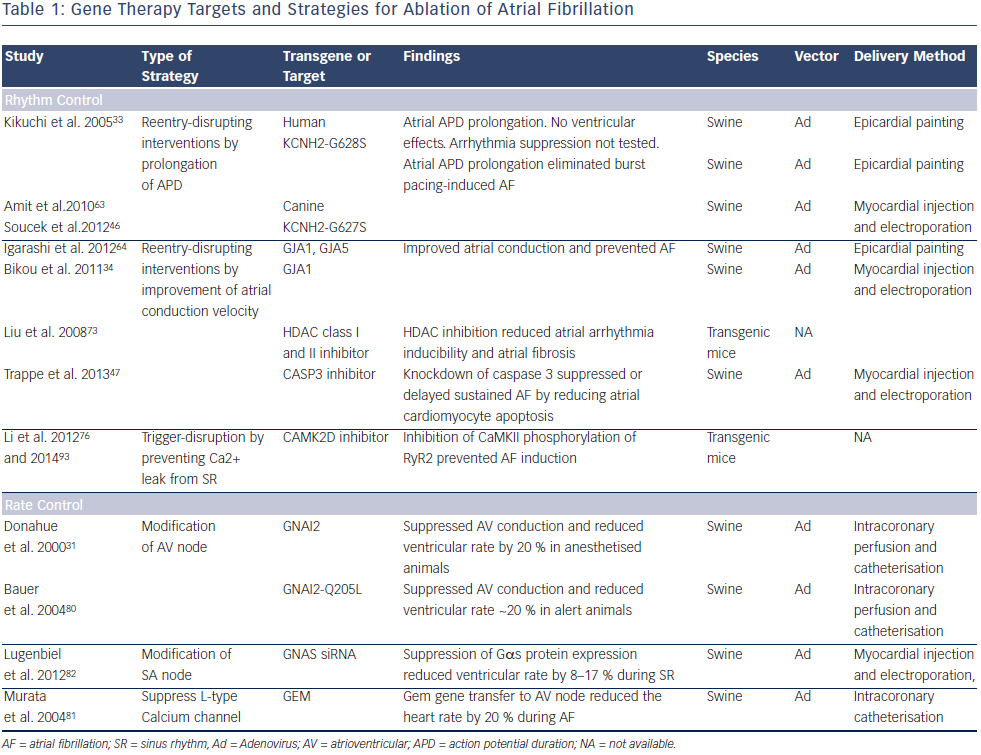
Gene Therapy Targets and Strategies for Ablation of Atrial Fibrillation
A key consideration for developing AF therapies is the arrhythmia mechanism. Both triggered activity and reentry have been implicated for provoking AF onset. Multiple lines of evidence support reentry as the dominant mechanism for sustaining AF.5,62 Approaches for sinus rhythm restoration include reentry-disrupting interventions such as prolongation of atrial action potential duration (APD), improvement of atrial conduction and trigger disruption by prevention of calcium leak from the sarcoplasmic reticulum. Rate control has targeted the AV node with overexpression of inhibitory G proteins, suppression of stimulatory G proteins, or introduction of a calcium channel inhibiting protein. Table 1 presents various gene targets and strategies for AF therapy.
Restoration of Sinus Rhythm
A principal element of AF electrical remodelling is the shortening of APD, which favours the maintenance of reentrant circuits. A logical approach would be to prevent AF by increasing the reentrant path length to prolong APD. Kikuchi et al. and Amit et al. demonstrated that gene transfer of the dominant negative mutation KCNH2 (G628S) blocked the IKr current and prolonged atrial APD.33 Animals receiving this gene by the epicardial gene painting method were resistant to atrial burst pacing-induced AF.63 The extent of APD prolongation and AF resistance correlated with gene expression. The dominant negative character of the mutation allowed it to suppress the endogenous, presumably normal, KCNH2 expressed in the atria. Soucek et al. performed a comparable study confirming that inhibition of KCNH2 function could prevent AF. They used the canine analogue of the channel (CERG-G627S) delivered to pigs using the injection/ electroporation method.46
A potentially complementary strategy is to prevent or reverse impaired intra-atrial conduction associated with AF or other diseases affecting the atria. One method to improve conduction is to target disease-related gap junction remodelling. Igarashi et al. found that atrial conduction impairment correlated with connexin (Cx) expression, phosphorylation and intercalated disk localisation. Using the atrial epicardial painting method, they showed not only that Cx43 gene transfer could reverse the conduction defect, but also that Cx40 gene transfer could replace the lost Cx43 and prevent AF.64 Bikou et al. additionally showed that Cx43 gene transfer using the injection/electroporation method improved atrial conduction and prevented AF.34
A possible approach to suppress conduction heterogeneity is to target atrial structural remodelling. Atrial apoptosis, inflammation and fibrosis are near universal findings, not only in AF, but also in diseases that support development of AF (e.g. HF, hypertension).65–67 Experimental studies indicate that AF can be prevented by suppression of fibrotic pathways including the renin–angiotensin system, transforming growth factor-β1, and other pathways relevant to inflammation and oxidative stress.68–72 As an example, histone deacetylase inhibition inhibited atrial fibrosis and reduced AF vulnerability in transgenic mice.73 No studies with gene therapy in clinically relevant models have yet been reported for prevention or reversal of atrial fibrosis, but this area holds promise.
AF is also linked to cardiomyocyte apoptosis, which leads to a reduction in conduction velocity. in vivo gene transfer with Ad-siRNA-Cas3 to knockdown caspase 3 has suppressed apoptosis, improved conduction velocity and delayed onset of AF, but didn’t alter myocardial fibrosis significantly.47
Calcium leak from the sarcoplasmic reticulum (SR) through ryanodine receptors (RYRs) potentially plays an important role in triggered activity that initiates AF.74 One strategy to target calcium leak from the SR is to reduce calcium/calmodulin-dependent protein kinase II (CaMKII) activity.75 Inhibition of CaMKII decreased phosphorylation of RyR2 and prevented induction of AF in FKBP12.6 knockout mice.76 Long-term inhibition of CaMKII prevented AF in CREM mice.77 A limitation of these data is that they are all from various transgenic mouse models. AF mechanisms are likely to differ in mice where triggered activity may play a more prominent role. Further investigations in preclinical models are required for a thorough understanding of the various roles of triggering and sustaining mechanisms in maintaining AF.
A strategy explored for protection against vagal-induced AF was administration of genes encoding the C-terminal fragment of Gαi and Gαo. The strategy competitively inhibited interaction between endogenous Gαi and Gαo with the muscarinic receptor, attenuating the effects of parasympathetic stimulation. Investigators delivered plasmids by direction injection followed by electroporation. Afterwards, they checked AF inducibility with vagal stimulation or carbachol administration. The combination of Gαi plus Gαo had similar effects on APD shortening when compared with Gαi alone, but had improved AF prevention effects suggesting a mechanism that involved more than the observed APD effects.18
Rate Control
AF normally results in an elevated ventricular rate. Rate controlling drugs are largely successful mainstays of therapy, but their use is limited in some patients by inadequate efficacy or intolerable side effects.78,79 Patients with AV node ablation are permanently dependent on pacemaker, and they have loss of synchronous left ventricular contraction that can be partially relieved by biventricular pacing, again requiring more implanted hardware. As a proof-of-concept, the inhibitory G-protein α-subunit (Gαi2) was incorporated into Ad and transferred to porcine AV node. The heart rate during AF after Gαi2 gene transfer reduced 20 %.31 In a follow-up study, the heart rate reduction with wild-type Gαi2 was lost when the animals were awake. A constitutively active Gαi2 mutation (cGi) caused a similar rate reduction that was impervious to animal arousal.80 A similar approach introduced Gem, an L-type calcium channel blocking G protein into the pig AV node and reduced ventricular rate.81
An alternative approach for AV nodal therapy is down-regulation of the stimulatory G protein α subunit. Lugenbeil et al. found that RNA interference-mediated inhibition of Gαs expression decreased ventricular rate by 20 %.82 In a separate study, they transferred this gene to the sinoatrial node and achieved an 8–17 % decrease in sinus rate.48 This strategy may well be complementary with the reported Gαi approach since the two proteins relay opposite signalling pathways in AV nodal cells. Gαs transmits β adrenergic signalling by increasing adenylate cyclase activity and downstream effects through protein kinase A. Gαi transmits cholinergic and purinergic signalling that decrease adenylate cyclase activity, antagonising the β-adrenergic effects in the AV node.
RNA interference is evolving to be a promising method for therapeutic silencing of protein-coding genes.83 MicroRNAs (MiRs) that inhibit expression of several proteins have been described. MiRs have been proven in various systems to be critical contributors to the pathophysiology of AF, either directly by modulation of ion channels and connexins, or indirectly by affecting fibrosis.84 MiR-1 is related to potassium channel and conduction velocity.85 MiR-21 and MiR-101 are associated with cardiac fibrosis.86,87 MiR-26 and MiR-328 contribute to the electrical remodelling of AF.88,89 MiRs are a potential gene therapy target for AF. This area is developing rapidly although it is still in an early stage.
Conclusions
Gene therapy approaches for AF are all currently at the preclinical development stage. Translation to clinical trial and practice is a long and difficult process. Clinical trials have demonstrated excellent longterm safety but limited efficacy for gene therapy in other cardiovascular diseases.22,23,60,61,90 The existing obstacles include lack of efficient, safe and clinically relevant delivery approaches and lack of vectors with high transfer efficiency and long-term regulated expression.91,92 Improvements in gene transfer vectors and delivery methods (including development of minimally invasive delivery methods) will further increase the likelihood of successful transfer of animal studies to clinical science. A better understanding of AF mechanisms in humans (particularly those with persistent AF complicated by heart failure or other cardiac diseases) is needed to refine therapeutic targets. Appropriate animal models with established AF need to be developed to study for longer time to insure effectiveness and durability of gene therapy. Overall, gene therapy for atrial fibrillation holds the potential to become the paradigm for clinical treatment but extensive further development is needed to reach this goal.