Catheter ablation is more effective than pharmacological therapy for the secondary prevention of patients with paroxysmal1,2 and persistent3,4 atrial fibrillation (AF) and has an emerging role in the primary prevention of paroxysmal AF.5,6 Nevertheless, in randomised clinical trials (RCTs) its success in treating patients with paroxysmal AF is 40–60 % for a single procedure and 70 % for multiple procedures at one year,1,2 and results for persistent AF are lower.7,8 Thus, there is an urgent unmet need to improve our understanding of mechanistic targets for AF in each patient, to match recent advances in ablation energy delivery and catheter positioning.
This report reviews mechanistic data on human AF, mapping technologies that provide the opportunity to reconcile fundamental mechanisms in individual patients and their therapeutic application.
Atrial Fibrillation Mechanisms
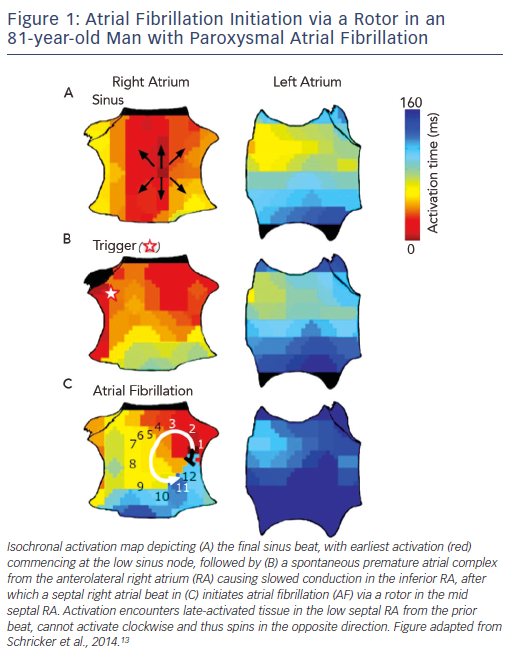
AF is initiated by triggers predominantly near the pulmonary veins (PV),9 with increasingly recognised sites outside the PVs.10–12 To improve success, ablation to eliminate AF triggers should thus also target non-PV triggers, although they are transient and difficult to locate. Another potential ablative target is the mechanistic cascade by which triggers initiate AF, yet this is relatively unstudied. We recently showed – using wide-area mapping during spontaneous and induced AF – that the first cycles of AF after a trigger exhibit a single organised re-entrant spiral wave or focal driver (see Figure 1)
that subsequently disorganises.13 Remarkably, these AF-initiating mechanisms may be relatively spatially fixed for each patient – even for different triggers from both atria – separated by 2.1 ± 1.7 cm from trigger sites (see Figure 1).13
Thus, AF initiation is likely to be a two-step mechanistic process, in which 1) a trigger initiates an organised spiral wave or focal source at patient-specific anatomical sites that 2) lead rapidly to disorganisation to produce the classical AF phenotype. This begs the question of whether AF maintenance is driven by self-sustaining disorganisation, or whether disorganisation is actually sustained by localised AF drivers (rotors or focal drivers).
Self-sustaining disorganisation was traditionally considered the mechanism of AF maintenance. This mechanism is supported by computational models in which multiple wavelets sustain AF if there is enough room in the atrium (‘critical mass’),14 proposals that dissociation between the epicardium and endocardium may be contributory15 and plaque recordings from the atria of patients in the operating room showing re-entry without apparent organisation. Limitations to these studies are that fibrillatory maps are based upon recordings that poorly represent local activation;16 only small areas of the atria were mapped (<10–20 %) and while these studies show that disorganisation exists they did not test if it self-sustains. The success of widespread ablation or Maze surgery supports the concept of self-sustaining disorganisation, but are also consistent with coincidental ablation of localised sources.
Localised sources for AF have been proposed for decades, supported by many clinical studies in the era of AF mapping and ablation.
This includes modulation or termination of AF by focal ablation or lesion sets insufficient to limit critical mass17,18 and anecdotes such as reproducible AF termination by catheter contact at one location.19 These observations directly support localised sources and contradict the concept of self-sustaining disorder. Indirect evidence for localised mechanisms include the spatiotemporal stability of AF,20 stable regions of high dominant frequency and consistent vectors of AF propagation.21 These models explain the success of hierarchical means of eliminating AF, rather than a probabilistic reduction in atrial mass alone.
Identification of Rotors in Human
Atrial Fibrillation
Focal impulse and rotor modulation (FIRM) mapping was first reported in 2011 to systematically and reproducibly identify localised drivers (rotors and focal sources) in human AF. Mechanistic studies demonstrate that such regions sustain human AF, clinical data report benefits for rotor ablation and multicentre RCTs are underway. Alternative techniques are increasingly available that reveal rotors and focal sources with many similarities but some differences to those identified by FIRM. While some differences may be explained methodologically, some will need pathophysiological studies to be reconciled. We summarise three basic principles that may have limited historical mapping of human AF, that were addressed specifically by FIRM and emerging mapping techniques.
Wide-area Mapping
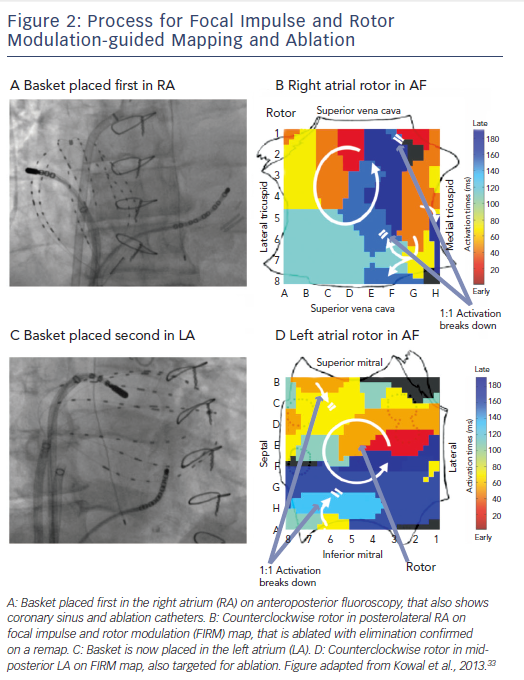
FIRM records AF in both atria sequentially using direct-contact
multi-electrode baskets (see Figure 2). Each basket comprises 64 electrodes, on eight splines of eight electrodes, advanced through the femoral veins to the right atrium (RA) and to the left atrium (LA) via transseptal puncture. Catheters are adjusted under fluoroscopic, echocardiographic and electro-anatomical guidance to optimise tissue contact.
Inter-electrode separation is 4–6 mm along each spline, with interspline separation of ~5 mm near poles and ~10 mm at equatorial electrodes. This is theoretically sufficient to map re-entry circuits in human atria whose minimum wavelength is ~4–5 cm.22 Direct electrode contact baskets overcome many limitations inherent to other mapping methods, such as potentially unreliable signals in AF from the inverse solution, limited mapping areas by surgical plaques, and toxicity from voltage-sensitive dyes. Nevertheless, improved basket resolution and compliance characteristics would further enhance mapping.
Separating Local from Far-field Activation
Intra-cardiac AF signals exhibit significant spatiotemporal variability, yet are more discrete using monophasic action potential (MAP) recordings than apparent from traditional bipolar or unipolar clinical recordings. In this sense, MAP signals may be closer to optically recorded signals based on human atrial repolarisation time (MAP duration) at different rates in different regions of both atria,23 how this alters signal analysis during AF24 and how it combines with rate-dependent conduction slowing25 to cause localised re-entry during AF.26 This has been the basis of physiological filtering employed in FIRM mapping to separate principal components from noise.27
Signal Processing Approaches to Identify and Track Precessing Rotors
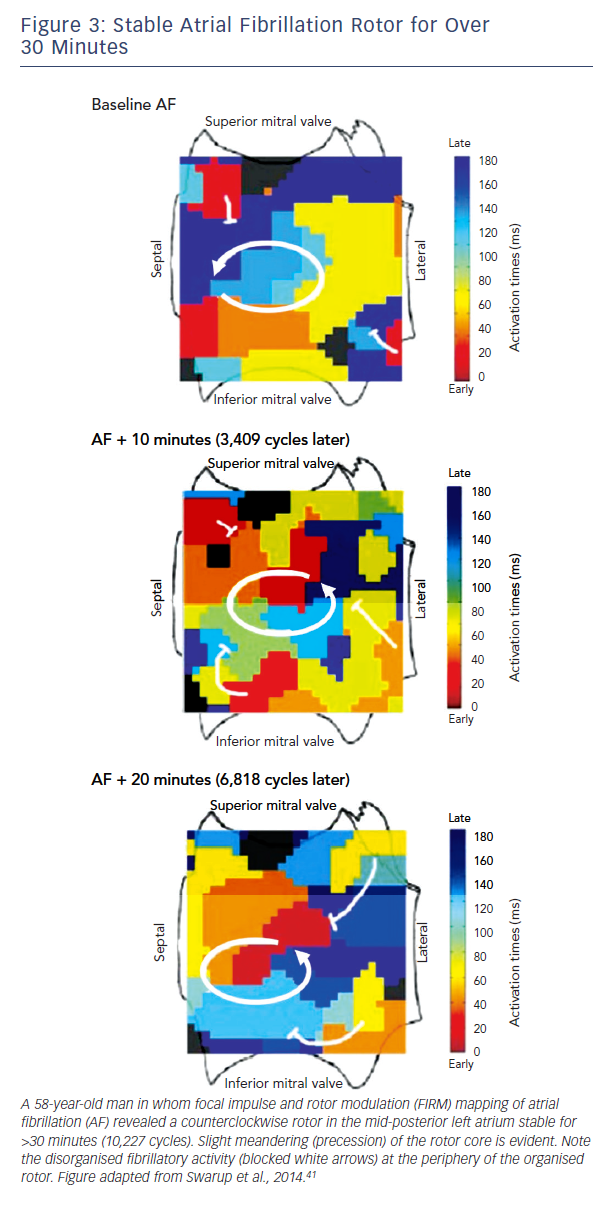
Traditional approaches to activation mapping are difficult to apply to AF. Firstly, rotors show precession (limited meander, see
Figure 3), a biophysical property of the core (phase singularity) that generates variable spiral arms that may not produce a classical rotational sequence on fixed electrodes. Precession also causes complex Doppler effects on local electrograms which, combined with disorganisation of spiral arms (fibrillatory conduction), makes activation mapping even more difficult.
In cell monolayers, rotor meandering causes fusion of action potentials to produce fractionated electrograms,28 that in clinical studies is further confused by the multiple technical causes of fractionated electrograms.29 These mechanisms may obscure rotors or give the illusion of rotors that are ‘unstable’ in activation maps, but are continuous if appropriate signal processing techniques are used.
Phase mapping is a signal processing approach that is increasingly utilised to identify rotors and track them as they precess. Phase mapping was developed in animal studies over two decades ago
using voltage sensitive dyes applied to action potentials.30 Put simply, phase mapping can identify AF rotors as sites where activation encroaches upon recovery – the core of a spiral wave. With a knowledge of local recovery time (repolarisation, action potential duration), rotors are identified as the crossing of lines of activation and recovery (phase singularities).
Differences Between Rotor Mapping Approaches
Since the demonstration of sustained human AF rotors in the Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation (CONFIRM) trial31 and multicentre FIRM registry,32,33 other investigators have shown localised rotational activity using PentaRay catheters,34 point-by-point wide-area mapping with wavelet similarity analysis,35 small intra-operative plaques36 and the inverse solution from body surface electrocardiograms (ECGs).37
Few studies have compared the characteristics of human AF rotors between mapping techniques. However, an emerging literature shows several similarities between rotor-mapping techniques. As with FIRM, newer techniques reveal a similar prevalence of rotational and focal sources (essentially all mapped patients with persistent and paroxysmal AF), location of sources in regions that are reproducible for hours or days in each patient, a similar split between right and left atrial sources (30/70 %) and improved ablative outcomes by targeting rotors.35
The most obvious stated difference is in rotor stability, with
inverse-solution ECG methods concluding less stable sources
than FIRM. Although differences are unlikely to be fully reconciled without direct comparisons to contact recordings in the same
patient, several factors may be at play. First, while rotors detected
by contact electrodes move with the atria during respiration or
systole, rotors detected on and referenced to the body surface
will of course be less stable when projected onto the moving heart.
The mere act of projecting electrograms from the heart to the
body surface may also magnify instability.38 Second, virtual electrograms from the inverse solution may not accurately depict contact electrograms in AF.39 Third, notwithstanding these
differences, a recent study using the inverse solution37 showed a small number of AF drivers regions that were temporally stable
(for days between mapping and ablation) in limited atrial regions targeted for ablation as in the CONFIRM trial.40 Finally, studying only short ECG segments between QRS complexes from the inverse solution37 may diminish stability evident when AF is mapped for longer periods.41 Re-mapping after rotor elimination may help to validate rotors that are physiological and eliminated by ablation, a central element of FIRM-guided ablation.
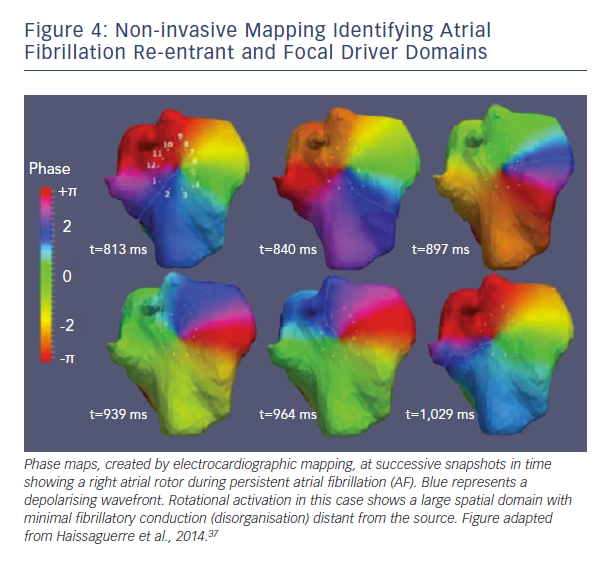
A second major difference between techniques is the spatial domain of organised spiral arms emanating from rotational sources. In FIRM maps, organised sources show spiral arms that disorganise via fibrillatory conduction (see Figure 3). Conversely, non-invasive phase maps often show organised activity (isolines) that often radiates across much/all of the atria from sources (see Figure 4). This is less consistent with fibrillatory conduction, and studies should determine if this difference represents smoothing from the body surface, algorithms used, or different definitions of sources.
Studies have recently proposed electrogram-similarity approaches as surrogates for human AF rotors.35,42 Due to precession, there may theoretically be no fixed location where electrograms represent a rotor core,30,43 but this interesting approach may identify regions related to AF sources that should be tested in clinical trials. In FIRM maps, precessing rotors in human AF produce electrograms that are regular in some cases and ‘fractionated’ in others.29
Approach To Focal Impulse and Rotor Modulation-based Rotor Ablation
Ablation of Atrial Fibrillation Sources
The principle for FIRM-guided ablation is that rotors should be targeted directly for ablation until they are eliminated on remaps. This is repeated for each AF source site with re-mapping to demonstrate progressive changes in AF.
In FIRM-guided ablation, sources are ablated directly over their precession areas. The reason for the direct ablation approach is historical: in early cases of FIRM, rotors in the mid-RA or mid-LA were targeted first as part of a planned line of ablation to non-conducting obstacles, but AF terminated before lines were completed. Accordingly, direct source ablation has become the default method, to cover the rotor precession areas of 2–3 cm2 (or 6–10 non-overlapping lesions of ~5–7 mm diameter).29 Many catheters have been used, including 3.5 mm tip open-irrigated radiofrequency (Thermocool, Biosense-Webster, Diamond Bar, CA), 8 mm tip non-irrigated radiofrequency (Blazer, Boston Scientific, Natick, MA), or cryoablation44 and robotic navigation.45 Typically two to three simultaneous rotors are identified within a given patient, requiring ~15–20 minutes of ablation covering 5-10 cm2 of atrial tissue. This area is less than conventional PV isolation (typically >40 minutes, or areas of >20 cm2), ablation of lines or complex fractionated atrial electrograms. FIRM ablation is commonly added to PV isolation but has been performed alone.
It is unclear how localised ablation eliminates rotors and different mechanisms may be at play in each patient. In computer models, ablation lesions placed within homogeneous atrial tissue will anchor an AF rotor to atrial tachycardia. Indeed, this result is commonly seen in FIRM studies. In more realistic non-uniform atrial models, ablation can terminate a rotor by several mechanisms including de-anchoring a rotor that meanders until it meets a non-conducting boundary and extinguishes, or eliminating regions of low excitability such that the wave front and back meet in remaining regions of high excitability and terminate. Ablation may also modify substrate more generally, such as by potential autonomic factors.
A major discussion point is why AF does not acutely terminate whenever sources are eliminated – and in general why AF does not have to be terminated by ablation to achieve clinical success.46 There are several hypotheses on this topic. First, it is possible that additional sources continue in unmapped regions of the atrium, that sustain AF acutely but are insufficient to sustain AF long-term. Second, AF may be a two-step process in which a driver interacts with fibrillatory conduction. The extent of time for which fibrillatory conduction
can sustain AF without a driver before ‘burning out’ is unclear, but may be seconds to minutes in control patients, or minutes to hours in AF patients with remodelled atria. Ongoing studies are addressing these mechanisms.
Clinical Results of Rotor Ablation
Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation Trial
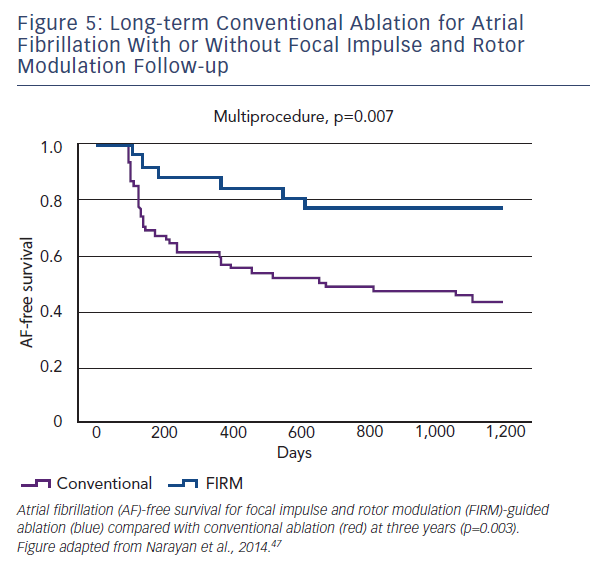
In this first trial of rotor/source mapping and ablation, 92 patients with drug-refractory AF underwent 107 consecutive ablation procedures. Cases were prospectively enrolled in one of two arms: FIRM-guided (34 %), whereby patients underwent FIRM-guided ablation followed by conventional ablation, or FIRM-blinded (66 %), who only underwent conventional ablation. The FIRM-blinded group still had FIRM mapping performed, offline, but was not used to guide therapy. The primary long-term endpoint was freedom from AF.
Localised sources were found in 97 % of all cases, with 2.1±1.0 sources per patient. Localised sources were mostly rotors (70 %)
and lay in widespread locations (76 % LA and 24 % RA). Long-term follow up revealed that freedom from AF in the FIRM-guided limb was higher than in the conventional therapy limb at one and subsequently at three years (77.8 versus 38.5 %, p<0.001) (see Figure 5).47
Focal Impulse and Rotor Modulation Outcomes in Multicentre Trials
Multicentre experiences in more than 30041 patients at over 10 independent laboratories support these results from FIRM-guided ablation.44,48 Notably as demonstrated by Miller et al.,44 even in largely persistent AF patients (71 %), FIRM-guided ablation achieved outcomes similar to those from CONFIRM, namely 80.5 % single-procedure freedom from AF, that trended higher in patients without prior ablation. Results were similar for persistent and paroxysmal AF (p=0.89). The additional recurrence from atrial tachycardias (including typical atrial flutter) in these studies has been approximately 10 %.
Clinical Data for other Rotor Mapping Approaches
Other approaches to target rotors have validated the importance of rotors as therapeutic targets in human AF, although with fewer data. The largest series to date is from the non-invasive ECG imaging approach, which recruited persistent AF patients with similar LA size to the FIRM guided group in CONFIRM.37 Ablation of rotational and focal ‘driver domains’ produced a similar freedom from AF at 12 months as the previously described stepwise ablation approach, but with less ablation time. Compared with the CONFIRM trial, this study had a higher rate of AT recurrences, which may reflect longer ablation or different patients, used different monitoring (thrice-monthly Holter monitoring versus implanted continuous monitoring in most FIRM-guided patients in CONFIRM) and more patients continued use of anti-arrhythmic drugs than in CONFIRM. Overall, this study confirms the importance of localised sources in persistent AF and their potential for localised ablative treatment.
Recently, results from a high-frequency source ablation (HFSA) approach showed non-inferiority at 12 months versus PV isolation (PVI), with improved safety outcomes.49 This builds upon solid translational research documenting sites of high dominant frequency (DF) as being responsible for driving AF50 but may differ from FIRM-guided rotor ablation as technical issues regarding DF interpretation51 and spatiotemporal stability52 may mean a meandering rotor may not precisely co-localise with the site of highest DF.
Limitations from Rotor Mapping Studies.
Both CONFIRM and other rotor mapping studies were non-randomised. The control populations in the non-invasive mapping study37 were from a non-consecutive historical cohort, which limits direct comparison. RCTs are underway, comparing source elimination with or without conventional ablation to conventional ablation alone. Endocardial mapping may also miss epicardial sources (and vice versa), or interpret intra-mural re-entry as focal breakthroughs, but currently simultaneous wide-area endocardial–epicardial mapping required to resolve such detail is not clinically feasible.
Clinical Implications
The identification of rotors that sustain human AF provides a novel paradigm for understanding AF mechanisms and offers a mechanistically focused patient-specific approach to catheter ablation. This is of particular significance for 1) patients with persistent AF, as recent data suggest that adding linear or complex fractionated atrial electrogram (CFAE) ablation does not improve the ~50 % success of PVI alone in patients with persistent AF53 and 2) patients with post-ablation AF, who often have isolated or minimally re-connected PVs at redo procedures. Further studies should compare rotors and regions of fibrillatory conduction between invasive and non-invasive approaches. Mechanistic studies should examine whether rotors lie near regions of atrial fibrosis detectable using magnetic resonance imaging (MRI) or ganglionic plexi, that may provide additive methods to image and target therapy. Notwithstanding the success of rotor-based ablative therapy in many single-centre studies, the results from ongoing multicentre trials will ultimately determine its wider role.
Conclusions
Improving outcomes for the growing number of patients with AF requires a deeper and unifying understanding of AF mechanisms. Identification of rotors sustaining human AF is now clinically feasible using available technology and a growing body of evidence shows that ablation targeting such rotors can improve long-term outcomes compared with conventional strategies alone. Rotor mapping and ablation may be of particular benefit in patients with persistent AF, in whom ablative targets are less clear. Results of multiple studies, including long-term data from CONFIRM, demonstrate that the patient-tailored therapy can reduce rates in patients with all types of AF, while reducing unnecessary ablation lesions. Multicentre RCTs are ongoing to better define the role of rotor ablation in the ablative treatment of persistent AF.