The main goal of mechanical cardiac assistance is to provide haemodynamic support in case of an endangered coronary or systemic circulation by increasing or maintaining coronary and systemic blood flow. In addition to haemodynamic support, mechanical cardiac assistance may also provide myocardial protection by unloading the ventricle. The experience with percutaneous mechanical support devices is mainly gathered in patients with cardiogenic shock (CS) or during high-risk percutaneous coronary intervention (PCI).
CS is a physiological state in which inadequate tissue perfusion results from cardiac dysfunction, most commonly due to acute myocardial infarction. Non-ischaemic causes include myocarditis, end-stage cardiomyopathy or sustained arrhythmias. CS remains the leading cause of death for hospitalised patients after ST-segment elevation myocardial infarction (STEMI).1 If CS occurs after STEMI, it is mostly a consequence of decreased myocardial contractility due to the infarction, resulting in a cascade of decreased cardiac output (CO), hypotension and decreased coronary blood flow, which will further reduce contractility and CO. This vicious circle may not only lead to further myocardial ischaemia, but also to diminished organ perfusion and ultimately multiple organ failure and death.
Patients with complex or high-risk coronary lesions due to extensive and diffuse multivessel, left main or last remaining coronary artery disease, who previously were not considered suitable for PCI, are increasingly treated with PCI. In patients who have been refused for cardiac surgery, PCI is increasingly considered as an alternative. During these high-risk procedures, haemodynamic compromise and complications can occur rapidly, for which support of a mechanical cardiac assist device can be helpful, particularly in patients with poor left ventricular (LV) function. Although the exact role of mechanical cardiac assistance in periprocedural risk management of complex and high-risk PCI procedures remains debatable, a growing number of high-risk PCI procedures are being performed with mechanical cardiac assistance.
Haemodynamic Support
The primary objective of cardiac support is the maintenance of haemodynamic stability. This is achieved by maintaining or improving coronary and systemic blood flow in order to ensure sufficient CO and adequate organ perfusion. The improvement of coronary and microvascular blood flow could also accelerate recovery of stunned myocardium after ischaemia. The SHOCK-trial investigators have shown that cardiac power output (CPO) is the best haemodynamic parameter to predict mortality in case of CS.2 This parameter takes the CO and the mean arterial pressure into account, based on the assumption that both adequate CO and blood pressure are necessary for sufficient end-organ perfusion. Haemodynamic support devices should be able to maintain both CO as well as blood pressure to provide adequate organ perfusion, ideally without the use of concomitant vasopressor or inotrope therapy.
Myocardial Protection
Some mechanical assist devices protect the myocardium by increasing oxygen delivery and reducing the oxygen demand, thereby preventing myocardial damage. This is achieved by a combination of increasing the aortic pressure and unloading the ventricle. Depending on the device, unloading is achieved through direct unloading of the left ventricle or by decreasing the preload of the left ventricle. Unloading the left ventricle results in a decreased LV end-diastolic pressure and peak LV wall stress, which in turn leads to decreased microvascular resistance, myocardial work load and myocardial oxygen consumption. By decreasing microvascular resistance, coronary blood flow is increased, as the latter is the result of the pressure difference between the proximal and distal vascular bed. In conclusion, the ideal cardiac assist device protects the myocardium by increasing coronary perfusion and decreasing myocardial workload.
Mechanical Assist Devices
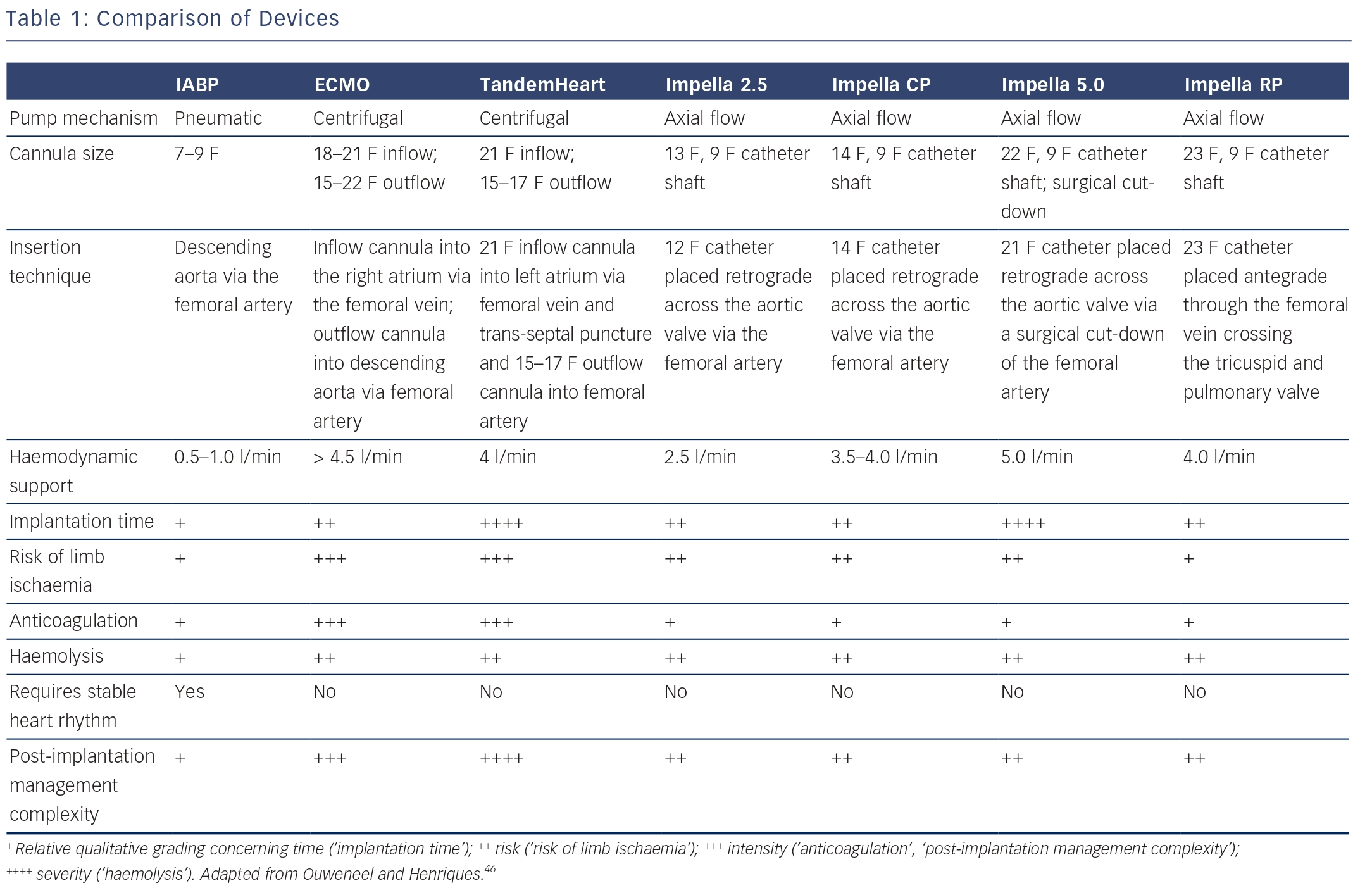
Over the past decades, many LV support devices have been developed, such as surgical bridge-to-transplant or destination therapy devices as well as temporary (percutaneous) bridge-to-recovery devices. During an acute critical presentation, a less-invasive percutaneous approach is preferable as it provides a quick and easy deployment. The ideal device is instantly accessible and should be associated with a low complication rate, as sometimes complications outweigh the potential beneficial effects. Complications associated with any (percutaneous) LV assist device include limb ischaemia, embolisation of atherosclerotic or thrombotic material, stroke, infection and haemolysis. In the following sections, the most common and currently used percutaneous devices are described: the intra-aortic balloon pump (IABP), TandemHeart (Cardiac Assist Inc., Pittsburgh, PA, US), ECMO (extracorporeal membrane oxygenation) and Impella (Abiomed Europe GmbH, Aachen, Germany). These devices differ in insertion technique as well as working mechanism.
Intra-aortic Balloon Pump
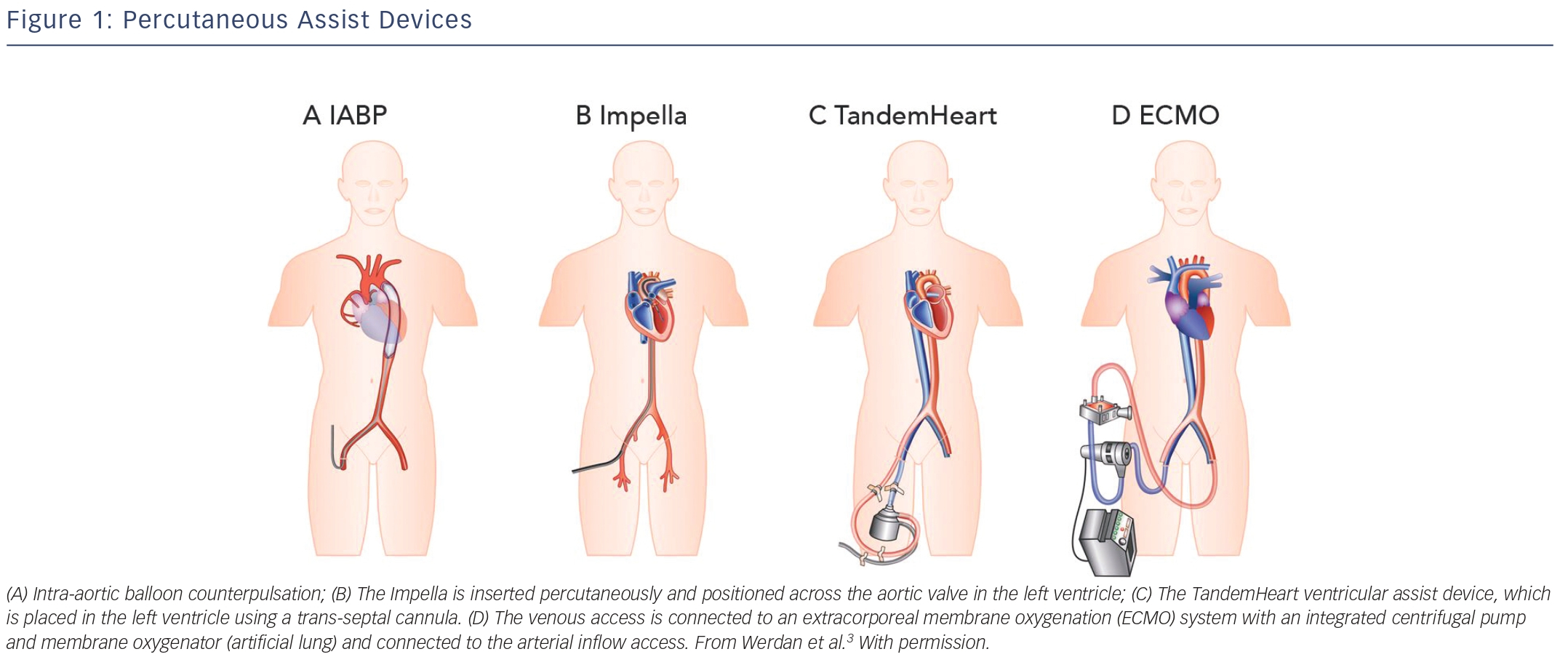
The IABP was, since its introduction in 1968, the most frequently used and broadly available cardiac assist device. The IABP is inserted percutaneously in the femoral artery and the balloon is positioned in the descending thoracic aorta distal to the left subclavian artery and proximal to the renal artery branches (see Figure 1A).3 The balloon is synchronised to the cardiac cycle and is rapidly inflated during diastole and rapidly deflated during early systole by input and removal of helium gas. The IABP is assumed to increase coronary and systemic blood flow due to augmentation of the diastolic blood pressure during inflation of the balloon. Deflation of the balloon decreases myocardial oxygen demand by decreasing afterload and ventricular wall tension, while slightly increasing coronary bloodflow.4 The IABP generates an increase of CO up to approximately 0.3–0.5 l/min. There are several limitations to the IABP. The augmentation of CO is likely to be insufficient for patients with severe CS. Also, to provide haemodynamic support it requires a certain level of LV function. Finally, the function of the IABP relies on synchronisation with the cardiac cycle, which might not be reliable in case of cardiac arrhythmias in critically ill patients.
Impella
The Impella is a micro-axial rotary pump that is placed across the aortic valve expelling aspirated blood from the left ventricle into the ascending aorta (see Figure 1B). Currently, there are three versions of the Impella system available. The Impella 2.5 and Impella CP can provide up to 2.5 l/min and 3.5–4.0 l/min, respectively, and are percutaneously inserted. The Impella 5.0 can deliver up to 5.0 l/min but requires a surgical cutdown of the femoral or axillary artery. The device has a pigtail-catheter at the tip to ensure stable positioning in the left ventricle and to avoid adhering to the myocardium. The direct unloading of the left ventricle is an important feature of the Impella. The unloading effect of the Impella 2.5 was demonstrated by a reduction in end-diastolic wall stress and immediate decrease in pulmonary capillary wedge pressure (PCWP).5–7 The Impella-induced increase in coronary bloodflow probably results from both an increased perfusion pressure and a decreased LV volume-related intra-myocardial resistance. In an experimental setting, haemodynamic support and unloading with the Impella has been demonstrated to reduce infarct size.8 The Impella 5.0 should result in even more unloading due to the substantially larger contribution to overall circulation. In contrast to the IABP, the Impella works independently of LV function and cardiac rhythm.
TandemHeart
TandemHeart is a trans-septal ventricular assist device that is inserted through the femoral vein and right atrium into the left atrium via an atrial septum puncture (see Figure 1C). The outflow cannula is inserted through the femoral artery and positioned at the level of the aortic bifurcation. It has a continuous flow centrifugal pump with a maximal rotation speed of 7,500 revolutions per minute, which can deliver up to 4 l/min. The haemodynamic effects of the TandemHeart are an increase of CO and mean arterial blood pressure (MAP) and a decrease of PCWP, central venous pressure (CVP) and pulmonary artery pressure (PAP). This results in reduced filling pressures in the left and right ventricle, a reduced cardiac workload and a lower oxygen demand.9–11 However, it should be noted, that without direct LV unloading, the increased MAP translates to increased LV afterload, which partially offsets the potential benefit of the lower cardiac workload. The main concerns are the complications (bleeding and limb ischaemia) and the complex insertion procedure. Percutaneous Extracorporeal Membrane Oxygenation ECMO can be achieved percutaneously and is a modified heart–lung machine, which can be used for several days (see Figure 1D). The ECMO system generally consists of a centrifugal pump, a heater and an oxygenator. Venous blood flows from the right atrium into a centrifugal pump and oxygenator and is guided via an outflow cannula in the femoral artery into the descending aorta. The advantage of ECMO over the other percutaneous devices is its ability to provide support in case of RV (RV) failure, to provide higher blood flow rates (up to 4.5 l/min depending on the cannula size) and to oxygenate the blood. Complications associated with ECMO are a systemic inflammatory response, renal failure, limb ischaemia and bleeding complications. Although ECMO can provide substantial haemodynamic support, it also increases both afterload and preload of the left ventricle, increasing the oxygen demand and impeding myocardial protection.12 However, patient transportation with ECMO is relatively easy, which makes it possible to start ECMO support outside the hospital.13
Right Ventricular Assist Devices
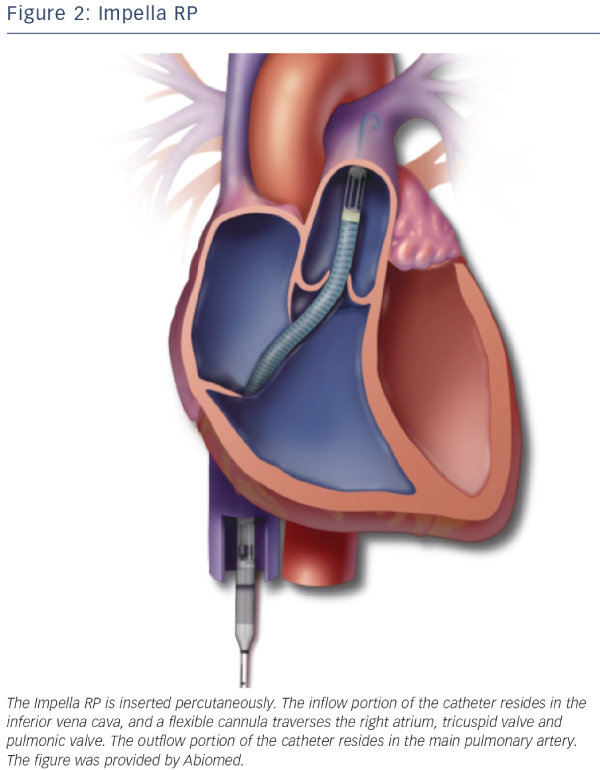
RV dysfunction has been shown to be a predictor of mortality in STEMI patients with and without CS.14,15 Although the majority of diagnostic and therapeutic approaches for CS are predominantly directed at the left ventricle, there are also assist devices that support the right ventricle. First of all the IABP has been thought to alleviate the RV but clear evidence is absent and due to its limited LV support it is unlikely that the IABP will assist the RV in a meaningful manner. The TandemHeart has an alternative set-up in which it can serve as a RV assist device.16 ECMO can also be used for RV or biventricular failure, as it displaces blood volume from the venous to the arterial circulation and oxygenates the blood. The disadvantage is that LV afterload is increased because there is no direct unloading of the left ventricle. The Impella RP, a specific Impella to support the right ventricle, has recently received a CE-mark in Europe. The Impella RP is placed percutaneously through the femoral vein and advanced in an antegrade fashion across the pulmonic valve into the pulmonary artery (see Figure 2). The Impella RP can provide flow up to 5 l/min for an anticipated duration of 14 days.17
Evidence
The current experience with percutaneous mechanical support devices is mainly in patients with CS or during high-risk PCI.
High-risk Percutaneous Coronary Intervention
During PCI, haemodynamic compromise and complications can occur rapidly, for which support of a mechanical cardiac assist device can be helpful, particularly in patients with poor LV function. Support of a mechanical assist device might be an appealing treatment strategy, however, iatrogenic complications due to this invasive therapy should not outweigh the potential benefits.
The exact role of mechanical support devices during high-risk PCI, the indication as well as the optimal device, is still open to debate. There is no generally accepted guideline-based definition for high-risk PCI, but it usually includes features such as a reduced LV function (ejection fraction [EF] <30 %), procedural complexity and procedural ischaemic risk, such as in left main PCI or last remaining vessel PCI.
When looking at the use of IABP in high-risk PCI, a few registries showed a potential benefit.18,19 Perera and colleagues performed a multicentre randomised controlled trial in high-risk PCI using prophylactic IABP placement before the procedure. They showed that usage of IABP did not reduce 28-day major adverse cardiac and cardiovascular events (MACCE).20 Patients undergoing elective IABP placement had fewer procedural complications, however, the rates of access complications and minor bleeding was higher. However, longterm follow up of these patients did show a difference in mortality.21
Regarding the use of Impella 2.5 during high-risk PCI, a few registries showing safety and feasibility of Impella 2.5 during high-risk PCI.22–25 There is one randomised controlled trial (PROTECT II), which showed no difference between Impella 2.5 and IABP on major adverse events on 30 and 90 days. However, this trial was prematurely discontinued for futility and therefore underpowered. Subgroup analysis showed significantly lower major adverse event rates in the Impella 2.5 group after excluding the first patients per group at each site, suggesting a learning curve associated with the initial introduction of the Impella 2.5.26 The learning curve shows that clinical trials should better address the training aspect of new devices, especially when compared with more established devices. The learning curve should also be taken into account when starting to work with a new device, especially in unstable patients. Increased utilisation of percutaneous assist devices in this elective setting will provide an important opportunity for operators to become familiar with these new devices. With increasing experience, operators also will feel more comfortable with the use in an acute setting.
Cardiogenic Shock
Intra-aortic Balloon Pump
Until recently, there were no large randomised controlled clinical trials investigating IABP therapy in the setting of STEMI complicated by CS. A meta-analysis of cohort studies published in 2009 concluded that there was insufficient evidence to support the previously strong guideline recommendation for IABP therapy in the setting of STEMI complicated by CS. The meta-analysis supported IABP therapy adjunctive to thrombolysis, but showed an increase in mortality for IABP therapy concomitant to primary PCI. More important the meta-analysis showed that the studied available observational data were importantly hampered by bias and confounding.27 A recently published Cochrane individual–patient–data meta-analysis of randomised controlled trials on patients with myocardial infarction complicated by CS, included six eligible and two ongoing studies with a total of 190 patients.28 The authors concluded that these few randomised trials were not able to show convincing evidence for either benefit of harm. The recently performed large randomised controlled multicentre IABP-SHOCK II trial randomised a total of 600 patients with STEMI complicated by CS, between IABP and medical therapy.29,30 No difference was found in 30-day all-cause mortality nor in other clinical endpoints. This trial confirmed the findings of the previously performed meta-analysis, showing no improved clinical outcome in patients treated with IABP concomitant to primary PCI in the setting of STEMI complicated by CS.
TandemHeart
Only a few small studies are performed using the TandemHeart but none of them were powered on mortality.9,10,31 These studies showed an improvement in haemodynamic values, mixed venous oxygen saturation and urine output and the two randomised controlled trials confirmed the superior improvement of haemodynamic parameters compared with IABP therapy. However, complications such as severe bleeding, arrhythmias and limb ischaemia occurred more often when using the TandemHeart than in cases of IABP. Although both studies were not powered to detect differences in mortality, no difference in mortality was found, which is also shown in a meta-analysis including both trials.32
Extracorporeal Membrane Oxygenation
Veno-arterial ECMO has been used successfully for several years in refractory CS, caused by myocarditis, postcardiotomy, myocardial infarction or heart transplantation.33 Described survival rates depend highly on the aetiology of the CS. In the setting of acute myocardial infarction, there are no randomised controlled trials. However, there are several cohort studies suggesting advantage from early use of ECMO.34,35 The usage of ECMO during resuscitation seems to increase, not only when resuscitation is performed inside a hospital, but also outside the hospital.13 The time from cardiac arrest to ECMO flow seems to be a critical determinant of outcome. However, no randomised trials have been performed yet.36
Impella
Only a few studies have reported on Impella usage in CS patients. Meyns et al. showed initial safety and feasibility in six patients with severe CS after maximal inotropic support and IABP therapy.7 They showed decreased PCWP and blood lactate levels and increased MAP and CO after usage of Impella. The ISAR-SHOCK randomised trial compared IABP with Impella 2.5 in CS patients.37 They showed increased cardiac index, CO and MAP in patients treated with Impella compared with IABP-treated patients. Also, they found that the overall cardiac power index (CPI) was slightly higher in Impella patients but the endogenous CO of the left ventricle was significantly lower because of the additional work of the device. Serum lactate levels were lower in the Impella group compared with the IABP group. No difference in mortality, major bleeding, distal limb ischaemia, arrhythmias and infections were found. The long-term effects of Impella support are described in the setting of STEMI-treated PCI and showed no aortic valve damage.38 Also, the Impella group patients showed more LVEF recovery compared with control patients. In severe CS, the Impella 5.0 may result in superior haemodynamic support. Engström et al. described the experience with the use of the Impella 2.5 and 5.0 and suggest that Impella 5.0 placement should be considered for profound CS patients.39 Either immediate insertion of Impella 5.0 or a quick upgrade to Impella 5.0 after initial 2.5 Impella was advised to be considered in cases with severe shock without signs of recovery. There are two ongoing randomised controlled trials evaluating Impella CP in the setting of CS: i.e. IMPRESS in Severe Shock (Impella CP versus IABP, NTR3450) and the Danish Cardiogenic Shock Trial (Impella CP versus optimal medical therpay, NCT01633502).
Combined Therapies
There are several cases describing a combination of ECMO and Impella therapy. It was found that the increased LV load due to the retrograde aortic blood return via the arterial cannula of ECMO could be counteracted by the use of Impella, which unloads the left ventricle.40–42 There have also been several reports on combining Impella with IABP therapy.43,44 However, the forward flow of the Impella may be degraded during diastole due to the diastolic pressure augmentation from the IABP. Although the combined use of IABP and Impella might theoretically increase coronary flow, due to a variety of technical reasons its combined use is not recommended. These reasons may range from misinterpretation of Impella alarms and the repetitious reductions of flow during IABP operation, which may prolong red cell transit times and thus increasing shear stress possibly leading to haemolysis. For patients with severe right heart failure, a combination of an Impella RP and a LV assist device has been described.17,45
Guidelines
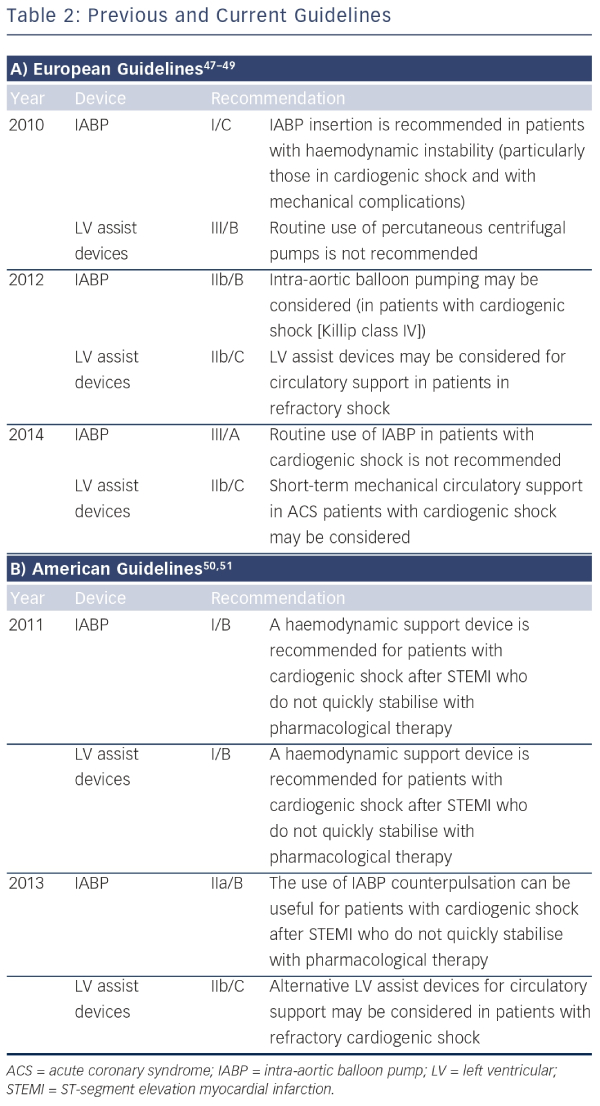
Recently, the guidelines on the role of mechanical circulatory support in patients with STEMI complicated by CS have significantly changed. The European guidelines of 2010 recommended the use of IABP (class I/C) in patients with CS, whereas the guidelines of 2014 do not recommend the routine use of IABP anymore (class III/A) (see Table 1).46 The main reasons for changing the guidelines are the publication of a meta-analysis and a large randomised controlled trial evaluating the clinical benefit of the IABP, both showing no beneficial effect on 30-day mortality.27,29 The 2013 US guidelines recommend to assess the need for inotropic therapy, IABP or both on an individual basis, as observational data are conflicting. The superior haemodynamic support of LV assist devices is mentioned, but with the annotation that the experience is limited. In these guidelines, IABP has a class IIa recommendation in patients who do not quickly stabilize with pharmacological therapy. Alternative LV assist devices may be considered in patients with refractory CS (class IIb). An overview of recommendations of using mechanical assist devices in CS is shown in Table 2.47–51
Future Perspectives
The experience of usage of mechanical assist devices is expanding rapidly. The indications include not only acute CS patients and highrisk PCI, but also as a bridge-to-transplant in advanced heart failure patients. In the forthcoming years, the development and the usage of percutaneous assist devices will increase and haemodynamic support will be used more frequently as an additional treatment in several patient groups. As recent studies were not able to show a survival benefit of the frequently used IABP in CS after myocardial infarction, the experience with other assist devices will expand in this setting. Other assist devices have not yet shown their benefit on survival, and are usually not available outside expert centres. As many attending cardiologists often feel the need to do more than just initiate pharmaceutical inotropic support, IABP will probably continue to be used until alternatives are widely available. However, it is important that other assist devices show clinical effectiveness before they become common clinical practice.
For usage of mechanical assist devices during high-risk PCI, there are two important questions that need to be answered. First, the question rises in terms of the role of mechanical circulatory support during these procedures. Is there a clinical benefit and, if so, in which patients? Second, which support device is the most optimal in this setting? The complications should not outweigh the possible clinical benefit of these invasive devices.
Future developments on mechanical assist devices need to focus on minimising insertion-point-related complications like limb-ischaemia and severe bleeding by reducing the device size while maintaining sufficient haemodynamic support. Also thrombo-embolic complications should be reduced and the associated morbidity needs to be minimised. New mechanical support devices, such as the pulsatile ECMO (Pulsecath) and the Reitan catheter pump (Cardiobridge), are being developed. The usage of percutaneous RV assist devices in case of RV failure is in development but only little experience is available.
Choosing the appropriate mechanical support device may depend on the degree of support needed. In consequence, subgroups of patients regarding the severity of CS have to be defined to allow a better discrimination between patient groups and devices to detect beneficial or harmful effects on outcome in different subgroups.
Conclusion
The ideal device generates sufficient haemodynamic support to prevent end-organ failure, but also myocardial protection to prevent myocardial ischaemia and has a low complication rate. The experience and usage of percutaneous cardiac assist devices in CS as well as high-risk PCI has increased over the past years. However, there is still little evidence of clinical benefit of these invasive devices and it is important that they prove their clinical benefit before they become common clinical practice.