Aortic valve replacement via full sternotomy is the gold standard surgical therapy for patients with severe aortic stenosis (AS) and insufficiency.1 This procedure has proved to be reliable, reproducible, relieves symptoms and improves prognosis of the patients. Degenerative AS is the most frequently acquired valve disease in the elderly population. In the current era, aortic valve surgery is the most common cardiac valve intervention in a cardiac surgery department.2
Improvements in anaesthesia, surgical techniques, post-operative care and in methods of myocardial protection has allowed surgeons to treat patients with increased age and or comorbidity safely with a low rate of morbidity and mortality. Data reported from the Society of Thoracic Surgeon (STS) database have shown a dramatic in-hospital mortality reduction from 3.4 % in 1997 to 2.6 % in 2006 for isolated AVR.3 The number of patients requiring aortic valve evaluation and intervention are increasing as the population grows and becomes older.4,5
However, physicians remain reluctant to recommend AVR for elderly patients more than 80 years of age or those considered very high risk.6 Instead, many patients are continued on medical management or undergo a balloon aortic valvuloplasty.6 Unfortunately, these conservative therapies provide minimal or short-lasting symptomatic relief to the patient, eventually leading to restenosis of the aortic valve or sudden death.
As a result, new techniques and technologies have been developed to enhance these outcomes, particularly in high-risk complex patients. As in other fields of medicine, a trend towards minimally invasive surgery has swept into cardiac surgery to achieve better results for the patients with the same quality as conventional median sternotomy.
The STS database defines minimally invasive cardiac surgery as “any procedure not performed with a Full Sternotomy and cardiopulmonary bypass (CPB) support”.7,8 The only aortic valve procedure precisely represented by this definition is transcatheter aortic valve implantation (TAVI). In this setting, TAVI offers an alternative treatment option in high-risk patients, having demonstrated to be superior to medical therapy in non-operable patients and non-inferior to surgical aortic valve procedure. However, controversies still exist regarding its effect on post-operative outcomes compared with conventional surgery. A meta-analysis of randomised, controlled trials that included 3,465 patients with severe AS found no significant differences between TAVI and conventional AVR in terms of myocardial infarction, stroke and mortality.9 Conversely, a sub-group analysis showed a higher incidence of vascular complications, neurological events, aortic regurgitation and need for permanent pacemaker implantation in patients undergoing TAVI.9
In 2008, a scientific statement from the American Heart Association defined minimally invasive cardiac surgery as “a small chest wall incision that does not include the conventional Full Sternotomy”; however, CPB is still utilised.10 The first description of aortic valve replacement (AVR) through right thoracotomy was published in 1993.11 Minimally invasive approaches through mini-sternotomy was popularised by Cleveland Clinic in 1996 and progressively spread in the surgical community around the world.12,13
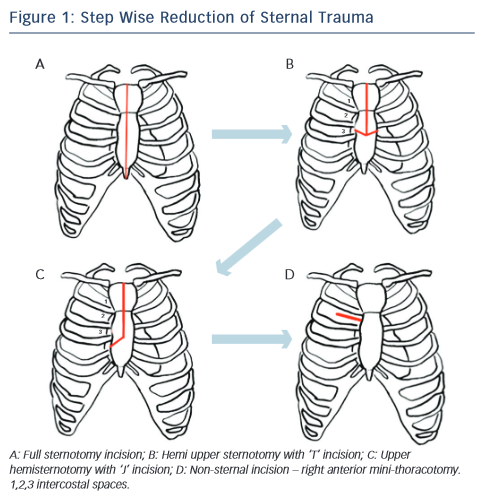
Historically different approaches has been described, and many types of classification, one as step wise approach for reduction of the trauma, from full incision sternotomy to non-incision sternotomy like right anterior mini thoracotomy (RAT) (see Figure 1). The most common techniques used today for minimal invasive aortic valve surgery (MIAVS) are RAT and upper hemisternotomy (UHS) incisions and hence those will be part of later discussion. Also, other approaches have been described, such as parasternal, transverse sternotomy and lower hemisternotomy.14–18
Different types of valves can be used, including standard mechanical and tissue valves. Stentless valve and sutureless valves can also be used in these minimally invasive approaches. Concomitant procedures, such as replacement of the ascending aorta and other valve interventions, have been described with these approaches as well.
Approaches
Minimally invasive aortic valve replacement (MIAVR) requires a coordinated effort by the surgeon, perfusionist, anaesthesiologist, cardiologists and nurses to achieve the best clinical outcomes. Intraoperative transesophageal echocardiography (TEE) is used routinely. A pulmonary artery catheter is employed based on patient risk and the specific operation. For both UHS and RAT, a single lumen endotracheal tube is standard. To achieve optimal exposure during RAT, the right lung can be mechanically retracted posteriorly without the need to resort to single-lung ventilation. However, right lung isolation can be useful during the learning curve and in difficult cases using double lumen endotracheal tube or bronchial blocker. To improve emptying of the heart during CPB, vacuum-assisted or kinetic venous drainage is commonly used. The patient is positioned supine and surgically prepped from the neck to mid-thigh for both procedures. External defibrillator pads are placed similarly to redo surgery. The mandatory use and routine interpretation of the intraoperative TEE for de-airing process is a critical step at the end of cardiopulmonary bypass in any MIAVS procedure. The echocardiogram images are visualised and evaluated at the actual time of the operation – this is performed in the same manner as in the conventional full sternotomy approach. Complete de-aired heart will allow weaning of cardiopulmonary bypass and transition to the end of the procedure reducing the microemboli phenomenon.
Upper Hemisternotomy
This is the most common incision used for surgeons for MIAVS. UHS may be the best approach for less-experienced surgeons. This approach implies to split the sternum, the sternotomy incision begins at the sternal notch and is carried down by 5–8 cm to the third or fourth intercostal space on the right. A sternal saw is used and the right internal thoracic artery is spared. A rigid retractor with narrow blades is inserted. Central aortic cannulation is straightforward but should be aimed as distal as possible to provide an unencumbered working space. Venous cannulation can either be peripheral or through the right atrial appendage. Myocardial protection is accomplished to the root or directly to the coronary ostia if antegrade is planned. Retrograde cardioplegia can be either directly or peripherally via internal jugular vein if required.18 The left ventricle can be vented directly through the aortic valve using cardiotomy suction or indirectly with a percutaneously placed pulmonary artery vent placed directly in to the pulmonary artery. A transverse aortotomy is placed slightly higher to facilitate its closure and visualisation at the end of operation (see Figure 2). Retraction sutures are placed on the edges of the aortotomy, and at the peak of each commissure to elevate the aortic valve into the centre of the operative field. The remaining steps of the procedure is similar to conventional valve replacement. We find that placement of the aortic valve sutures is facilitated by instruments with long handles and also using a knotting device such as CoreKnot® reduces valve implant time. As the surface of the heart is not readily accessible, de-airing demands meticulous attention to detail and is monitored using TEE, being as described above a critical step in the operation.
Right Anterior Mini Thoracotomy
RAT avoids sternotomy and is associated with a limited skin incision. However, the operative field is smaller and the aortic valve sits deeper within the wound. Exposure is enhanced by minimising cannula traffic within the incision via peripheral access, coupled with strategic placement of pericardial sutures. This approach is typically performed with a 4–6 cm incision through the second or third intercostal space.
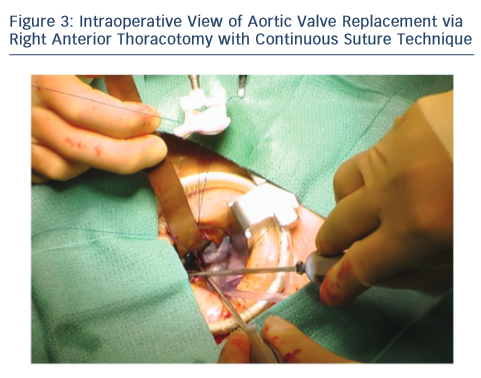
Upon entering the pleural space, the right mammary vessels are usually ligated and divided. The third or fourth rib can be dislocated from the sternum to enhance exposure with the goal of visualising the tip of the right atrial appendage. A soft tissue retractor is inserted into the wound followed by a rigid retractor with narrow blades (see Figure 3). Cannulation for cardiopulmonary bypass can be central but usually peripheral. The crossclamp is applied directly through the incision or from an alternative port; however, it can also be performed peripherally with an endoclamp. Myocardial protection with antegrade cardioplegia is delivered through the root or directly to the coronary ostia. Retrograde cardioplegia could be also delivered peripherally through percutaneous jugular vein catheter into the coronary sinus. Technical details of aortotomy, prosthetic valve implantation and aortotomy closure are identical to UHS. The aortic valve is excised in the usual fashion; however, the right coronary cusp sutures are placed first and retracted to facilitate exposure. At the end of the procedure, a small chest drainage tube (e.g. Blake) is inserted in the right pleural space through a separate intercostal space. Pericardium is left open. The disarticulated rib is reattached to the sternum using non-absorbable, braided suture. To avoid lung herniation, the ribs are then reapproximated using further non-absorbable braided sutures.
Importantly, if exposure with either UHS or RAT is inadequate, then conversion to full sternotomy should be considered. This ensures that valve replacement can be completed safely using an approach familiar to the surgeon.
Advantages and Disadvantages
Randomised trials comparing conventional sternotomy to MIAVR face formidable challenges because of patient preference, surgeon bias and, importantly, the lack of a standardised surgical approach. Postoperative complications associated with a full sternotomy are practically possible with minimal invasive approach16 In theory, avoiding full sternotomy should contribute to better post-operative stability of the sternum and thereby prevent deep infection and preserve respiratory function and mobility in the immediate post-operative period. A smaller area of exposed sternal bone marrow and periosteum may also minimise bleeding. Several retrospective studies have shown that MIAVS reduces exposure of surgical trauma to the patient, post-operative pain, blood transfusion, risk of renal failure, times for mechanical ventilation and, therefore, reduces intensive care length of stay. The hospital postoperative length of stay is also diminished.19 Patient satisfaction and recovery to normal physical activity is also improved.5,20–22 Murtuza et al. published a meta-analysis of MIAVS versus conventional AVR studies. They included over 20 studies consisting of more than 4,000 patients.22 MIAVR was associated with a significant reduction in mortality, shorter intensive care unit (ICU) and hospital lengths of stay and decreased ventilation times and transfusion rates.22
However, MIAVR was also associated with longer myocardial ischaemic, cardiopulmonary bypass and operative times compared with open procedures as a result of the steep learning curve involved, especially at the earliest stages of training.22 Since MIAVR procedures are usually technically more demanding, some surgeons argue that no compromises in quality should be allowed for the purpose of a smaller incision. It was also argued that de-airing at the end of such procedure could be incomplete.
An important fact to emphasise is that the outcome and quality of the procedure are comparable or superior to the conventional open or full sternotomy procedures, including the risks of cerebrovascular events. The recent introduction in the market of balloon expandable sutureless valves has enable a reducion of these times.
Another potential disadvantage of MIAVR is the morbidity associated with peripheral cannulation, which may cause wound infection, pseudoaneurysms and neurological events. Nevertheless the improvements in technique over time has decreased the morbidity of the procedure and allows surgeons to perform the procedure in high risk and elderly patients as more familiar approach and even better-than-predicted survival in this population.5 However, despite these procedures being potentially more expensive compared with full sternotomy procedures, the benefit is proven and it leads to a reduction in post-operative complications, shorter hospital stay and faster recovery, which should result in lower costs in the long term.
Preoperative Planning
Multidisciplinary preoperative and detailed planning allows better outcomes for patients. Essential and reproducible plannification is primordial for an efficient treatment.18 Effective preoperative planning is essential to identify any further complications prior to surgery that could delay patient recovery.
Preoperative conditions such as chronic lung diseases, cerebrovascular disease, peripheral artery disease and chest wall abnormalities, lung irradiation and previous cardiac/lung surgery are specially emphasised within these minimally invasive approaches.
Routine preoperative evaluation test such as electrocardiogram, chest X-Ray, complete bloods laboratory tests, echocardiogram and angiogram are performed in the usual manner for full sternotomy counterparts. However preoperative investigations could differ slightly from the routine investigations for standard AVR.
Computed tomography (CT) has an important role in the preoperative study for these minimally invasive procedures. CT allows better understanding of the anatomy and the safer delivery of either procedure. The CT gives us information about the lungs, airway, chest wall and mediastinum, including heart and great vessels. Different entities will preclude a challenging but not impossible procedure, such as lung adhesions, diaphragm paralysis and chest wall abnormalities with kyposcolisosis, pectus carinatum or pectus excavatum. Those pathologies might change the initial planned approach. In patients with previous cardiac surgery or chest wall irradiation, a chest CT conveys the distance between the posterior sternal table and right ventricle. The presence of patent coronary bypass grafts crossing the midline is particularly hazardous. For the UHS approach, CT scan confirms to which intercostal space to extend the J.
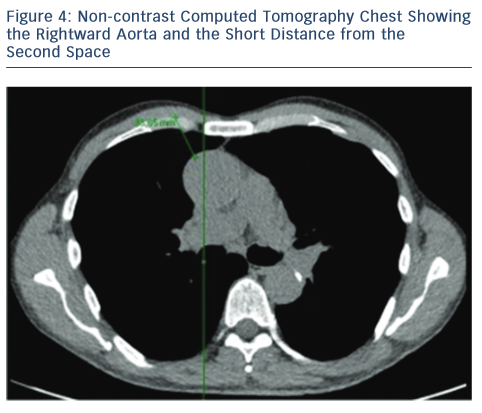
For the RAT approach, the CT scan also facilitates important information regarding the aorta and the relationship with the sternum. By noting which intercostal space is closest to the tip of the right atrial appendage, the preferred intercostal space is identified during the RAT approach. In essence, the RAT procedure is more favourable if:
- the aorta lies more than one-half to the right of a vertical line drawn from the right sternal border to the ascending aorta in the axial CT view; and23
- the distance is less than 10 cm from the skin to the ascending aorta where the pulmonary artery bifurcates (see Figure 4).23
Peripheral vascular and cerebrovascular disease increases the risk of stroke and embolisation. Careful assessment of the vascular system is carried out. CT angiogram is performed if suspicious or elevated risk of stroke or embolisation due to retrograde perfusion through peripheral cannulation is anticipated. Arteriosclerosis and calcium plaques in the aorta help us to choose different strategies for cannulation sites. Smooth, calcified plaque is less hazardous than soft or irregular plaque. In addition, the relative size and tortuosity of the iliofemoral vessels on angiogram are important factors in selecting the appropriate arterial cannula. Sealant devices such as angio-seal® are not recommended after preoperative angiogram because it will be difficult to perform femoral cut-down and subsequent cannulation in the procedure. In a patient with a history of stroke or transient ischaemic attack, duplex scanning of the carotid and vertebral arteries is obtained.
Hybrid Procedures
As growing expertise and number of procedures performed minimally invasive, the hybrid procedures are being explored. Pre-existing coronary disease does not contraindicate minimally invasive approaches as hybrid or staged procedures can be performed with good and comparable results. Different studies have evaluated the safety and benefit of these procedures. However, further prospective randomised controlled trials needs to be addressed to clarify which is the better approach whether staged/hybrid percutaneous coronary intervention (PCI) and AVR via minimal invasive or full sternotomy combined procedure AVR and coronary artery bypass graft.24
Discussion
With an increased population and, in turn, life expectancy, it could be anticipated the older generation will continue to grow. The elderly patient inevitably will have multiple pathologies. Nowadays complex cases and high-risk patients need to be approached with the most recent available techniques. Over the past 2 decades minimally invasive aortic valve surgery has been gradually introduced into clinical practice. The increasing popularity for less-invasive procedures allow surgeons to perform complex cardiac interventions with the same quality even with smaller incisions. Overall, minimally invasive surgery and combined procedures or staged/hybrid procedures permits good outcomes, even in high-risk populations.25
In today’s society of patient care is expectated to be at an increasingly high standard. Moreover, patients’ requests include minimally invasive procedures. Patient choice is more contemplated, evaluated in the current practice and high on the agenda in the healthcare setting. Consultation with the patients should thus include the option of a minimally invasive approach as routine. Cardiac surgeons and cardiologists must provide the most effective treatment for their patients – as physicians we need to learn to adapt to the new changing techniques. Nevertheless, safety and quality of life of patients must never be compromised and should be the first priority above any marketing concerns. It is necessary to adopt and learn these techniques in the armamentarium of treatment of heart valve disease.26 Essential endovascular skills are necessary for cardiac surgeons, therefore close communication with an interventional cardiologist is mandatory.
The drawback for MIAVS is increased cardiopulmonary bypass and crossclamp times and is therefore technically more demanding for surgeons. The longer learning curve also can be detrimental for the adoption of these newer techniques. Despite these factors, the benefits shown in different retrospective studies are greater, such as improved cosmesis, reduced post-operative pain, reduced blood transfusion, reduced ventilator times and hospital length of stay.26 In order to reduce intraoperative times, three different sutureless or rapid deployment aortic valves have been recently introduced in Europe for use in both conventional AVR and MIAVR operations – the Enable™ Valve System (Medtronic, Minneapolis, MN, USA), the Perceval S™ Valve System (Sorin Biomedica Cardio Srl, Sallugia, Italy) and the Edwards Intuity™ Valve System (Edwards Lifesciences, Irvine, CA, US). In a recent study of patients undergoing MIAVR approach and sutureless devices, Santarpino et al. showed better outcomes in the sutureless group, suggesting that the combination of a MIAVR associated with a sutureless valve may be the first-line treatment for high-risk patients considered to be in the grey zone between TAVI and conventional surgery.27 Gilmanov et al. published a series of 515 patients undergoing RAT AVR, 269 with conventional prostheses and 246 using sutureless prostheses.28 They showed that CPB and crossclamp time was significantly shorter in the sutureless group, while peri-operative strokes, pacemaker implantations and in-hospital mortality were comparable.28 At median follow-up of 21 months, there was a twofold higher actual survival in the octogenarian patients with sutureless compared with sutured valves (100 % versus 50 %; P=0.02).28 We believe that sutureless valves and transcatheter procedures will be become more prevalent as part of everyday practice in the present and in the future.
We have already mentioned studies comparing outcomes of MIAVR with conventional AVR. However, there is little evidence comparing the outcomes of the UHS versus the RAT approach. Miceli et al. retrospectively examined AVR in 406 patients by either RAT or mini-sternotomy and found that patients who received RAT experienced reduced ventilation time (median 7 hours, interquartile range [IQR] 5–9 hours versus median 8 hours; IQR 6–12 hours; P=0.003), a lower incidence of new-onset postoperative atrial fibrillation (AF) (19.5 % versus 34.2 %; P=0.01), shorter ICU stays (median 1 day, IQR 1 day versus median 1 day, IQR 1–2 days; P=0.001) and overall hospital stays (median 5 days, IQR 5–6 days versus median 6 days, IQR 5–8 days; P=0.0001) compared with mini-sternotomy patients.29 In addition, survival at 1 year and 5 years was higher for RAT patients relative to mini-sternotomy patients (97 % and 86 % versus 94 % and 80 %; P=0.1), although the difference was not statistically significant.29 Similiarly, in a propensity score matched analysis, Hiraoka et al.29 found that RAT patients experienced fewer blood transfusions (42 % versus 67 %; P=0.025), a shorter operative time (235 ± 35 minute versus 272 ± 73 minute; P=0.009), shorter ICU stays (1.4 ± 0.8 days versus 2.2 ± 1.1 days; P=0.001) and shorter hospital stays (13.3 ± 6.5 days versus 21.5 ± 10.3 days; P=0.001, respectively) compared with partial and full sternotomy patients.30 Furthermore, patients who undergo RAT have little to no post-operative physical restrictions because the sternum is left intact and stable during surgery. This is in contrast to patients undergoing a UHS who are required to take sternal precautions after surgery. Larger, randomised controlled studies are needed to compare the efficacy and benefits of the two methods in detail.
Future techniques as robotic and video-assisted surgeries are not as distant and inaccessible techniques were in the past decades. In order for this to be achievable, more education, funding and training needs to be provided routinely. Furthermore, as clinical trials continue with transcatheter valves, if MIAVS continues to demonstrate superior outcomes compared with full sternotomy, then it should be assumed that MIAVS should be the golden standard used to compare these emerging technologies against.
Conclusions
To summarise, minimally invasive aortic valve surgery is safe and reproducible. Fewer complications are likely with a detailed and selective as appropriate plan of preoperative investigations. There is significant evidence to suggest that a shorter post-operative stay and reduced number of complications, such a blood loss and post-operative pain, are associated with minimally invasive procedures. Despite a longer learning curve and challenging procedures the improved outcome gives to the patient the optimum chance of faster recovery with the return to normal activity. Minimally invasive aortic valve procedures should be offered to any patients deemed appropriate to benefit to this approach. Ultimately, adoption of minimally invasive cardiac surgery will enhance professional careers of cardiac surgeons as well as the lives of their patients.