With the increasing use of contrast agents for both diagnostic and interventional procedures, contrast-induced acute kidney injury (CI-AKI) has become an important medical concern. It is estimated that CI-AKI is the third most common cause of hospital-acquired renal failure and has significant prognostic implications for patients outcomes.1 The incidence of CI-AKI varies depending on the definitions and cut-off values used. Although serum creatinine is subject to fluctuation during the hospital stay and is influenced by multiple factors, it is an easy, cost-effective way to estimate kidney function and therefore it is usually used to determine CI-AKI.2 The most common definition of CI-AKI comprises an absolute increase in serum creatinine levels of ≥0.5 mg/dl (44 μmol/l) or a ≥25 % relative rise from baseline within 72 hours from contrast media (CM) exposure. Importantly, alternative etiologies for kidney injury, such as microembolism or severe hypotension should be excluded. Overall, the incidence of CI-AKI in the general population is reported to be 0.6–2 %.3 However, the prevalence is higher in patients undergoing coronary angiography and percutaneous coronary interventions, most likely due to high CM volumes used during these procedures and the type of patients treated, often presenting multiple comorbidities.1,3
A sub-analysis of the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) and Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) randomised clinical trials has reported a frequency of CI-AKI of 12.5 % after percutaneous coronary interventions (PCI).4 In older patients with preexisting renal dysfunction, particularly if associated with diabetes or congestive heart failure (CHF), the frequency of CI-AKI further rises to be >20–30 %.3,5,6
Natural History of CI-AKI
While in most cases CI-AKI is self-limiting and creatinine or estimated glomerular filtration rate (eGFR) return to baseline levels in 5–10 days, in patients with a high risk profile for kidney damage, CI-AKI is associated with increased rates of in-hospital and short-term outcomes. Rihal et al. observed a dramatically higher rate of in-hospital mortality in patients undergoing PCI who developed CI-AKI (22 % versus 1.4 %; p<0.0001).7 Similarly, Form et al. reported that CI-AKI, following contrastenhanced procedures, was associated with an increased risk for 30-day mortality after adjustment for potential confounding variables (odds ratio [OR] 3.37; 95 % confidence interval [CI]; [2.58–4.41]).8 Even small increases of serum creatinine, greater than 0.25 mg/dl but lower than the commonly used threshold of 0.5 mg/dl, after coronary angiography seem independently associated with prolonged in-hospital stay and with increased in-hospital mortality.9 In addition to short-term complications, CI-AKI can have repercussions on longterm renal function and can precipitate chronic kidney disease (CKD) progression.10 An observational study has recently estimated that patients with CI-AKI have a 4–17-fold higher risk of renal impairment at 3 months after index PCI depending on the severity of post procedural CI-AKI.11 Moreover, CI-AKI has been associated with significantly higher mortality rates at 1 year (9.8 % versus 2.9 %; p<0.0001) compared to patients undergoing uncomplicated PCI. Similarly, the 1-year rate of myocardial infarction (MI), definite/probable stent thrombosis, target lesion revascularisation and major bleeding (13.8 % versus 5.4 %; hazard ratio [HR] 2.64 [2.21–3.15]; p<0.0001) were also higher in patients with CI-AKI, even after multivariable adjustment.4 Consistent with these results, Solomon et al. demonstrated a greater than threefold rise in adverse events (death, stroke, MI, end-stage renal disease requiring renal replacement therapy) at 1 year after angiography in patients with CI-AKI defined with a lower cut-off threshold of 0.3 mg/dl of absolute creatinine increase.12 Finally, CI-AKI has also been linked to higher 5-year mortality rates after PCI. Despite the difficulty to account for the progression of comorbidities and other risk factors that could explain the different mortality rates between patients with and without CI-AKI, results have been consistent across various prospective studies.7,13,14 Therefore, implementation of preventive measures is crucial to reduce frequency of CI-AKI and avoid short- and long-term clinical outcomes.
Pathophysiology of Contrast-induced Nephropathy
CM is almost completely eliminated by the kidney approximately 30–60 minutes after administration. The mechanisms of CM nephrotoxicity are complex and not fully understood. However, for the purpose of this review the process can be simplified into three main components: parenchymal ischaemia, direct and indirect tubular cell injury and direct and indirect damage of the vascular endothelium. In brief, upon administration of CM, a transient vasodilation with increase in renal blood supply has been observed, quickly followed by a reduction of blood flow and GFR.15 The mechanisms involved include decreased production of vasodilating agents such as prostaglandin and nitric oxide (NO), impaired endothelial function and vasoconstriction of the vasa recta.16 The ensuing parenchymal ischaemia becomes more relevant in the outer medulla where oxygen delivery is scarce even in physiological conditions because of the anatomical distribution of the vasa recta.16,17 As a result of renal hypoxia, reactive oxygen species (ROS) are produced that then can damage both the tubular cells and the vascular endothelium.18,19 In addition, the CM filtered by the glomeruli is concentrated in the tubular lumen through extra-tubular fluid reabsorption.20 Direct contact of CM can injure tubular cells and induce apoptosis through the activation of shock proteins.21–23 These effects vary depending on the osmolarity and ionic strength of the CM.19 Furthermore, CM increases urinary viscosity in the tubules and can determine slow flow, thus prolonging the exposure of tubular cells to the CM, and high intratubular pressure that further exacerbates medullary ischaemia by compressing the vasa recta.20,24 Additionally, the slow blood flow in the restricted vasa recta will increase the contact time of CM with the vascular endothelium contributing to its damage.
Risk Factors
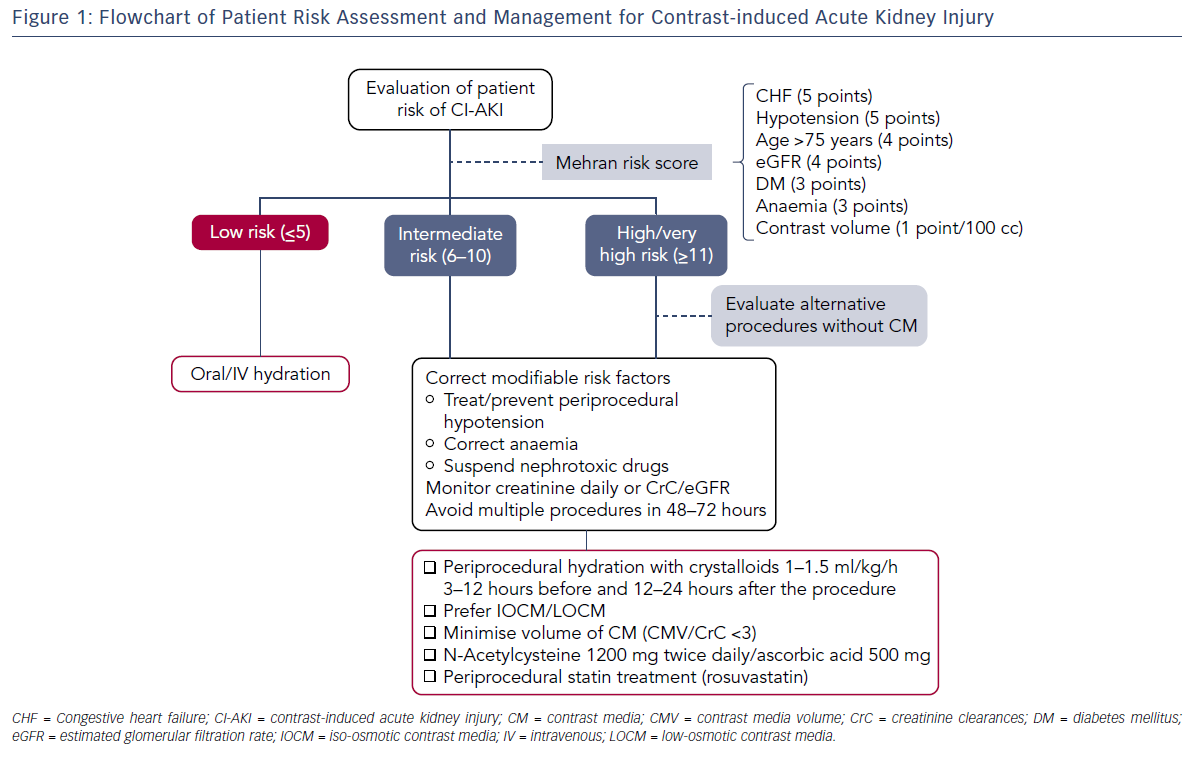
Risk scores have been developed to predict CI-AKI and identify patients who would benefit from preventive measures. The Mehran model includes both clinical and peri-procedural risk factors: congestive heart failure (CHF) (5 points), hypotension (5 points), eGFR (4 points), age >75 years (4 points), diabetes (3 points), anaemia (3 points) and contrast volume (1 point for every 100 cc used). Based on the overall score patients can be stratified into four different risk categories: low (score ≤5) moderate (score 6–10), high (score 11–15) or very high risk (score ≥16).25 Similarly, Brown et al. have validated a model consisting of pre-procedural variables only, such as serum creatinine, CHF, diabetes, urgent or emergency priority, intra-aortic balloon pump use, age ≥80 years and female sex.26 Both models identify fixed and modifiable risk factors with addictive effects on the incidence of CI-AKI, kidney failure requiring dialysis and mortality (see Table 1).
Preexisting kidney impairment is the main risk factor for CI-AKI. A decreased vasodilatory response has been observed in the available nephrons of CKD animal models with a consequent deficiency of medullary oxygen that would predispose to the ischaemic damage observed in CI-AKI.27 In patients with CKD, Moore et al. and Barrett et al. described an increase from 4 to 20 % in the incidence of CI-AKI as the baseline serum creatinine level rose from 1.2 to 2.9 mg/dl.28 In addition, a larger decrement in eGFR 2 years following the procedure has been observed in patients with CKD who experienced transient CI-AKI after coronary angiography (eGFR at 2 years, -20 ± 11 ml/min 1.73 m2 versus -6 ± 16 ml/min/1.73 m2; P=0.02).13 Since accurate determination of kidney function is so critical in CKD patients undergoing CM exposure, direct measurement of the creatinine clearance (CrC) or estimation of GFR with the MDRD equation is preferable to serum creatinine in order to assess renal function. CrC or eGFR should be estimated in high-risk patients before the procedure and at various points during the post-procedural follow-up.
Diabetes and hypertension both contribute to CKD. Even in the absence of clinically diagnosed CKD, endothelial dysfunction with reduced production of NO and vasoconstriction of vasa recta can be observed in patients with hypertension and diabetes and can contribute to the development of CI-AKI.29 Furthermore, preprocedural hyperglycaemia in patients without diabetes has been associated with higher rates of CI-AKI.30 The mechanism involved is based on the regional glucose catabolism that increases lactate concentration in the renal medulla and consequently promotes acidosis and production of ROS upon CM administration. When present, CKD with a cut-off value of GFR <60 ml/min is the strongest predictor of CI-AKI in diabetic patients. Incidence of CI-AKI after coronary angiography has been reported to be around 16 % in patients with diabetes and preserved renal function but it reaches 38 % in diabetic patients with CKD. In addition, in patients with CKD and diabetes, CI-AKI is a strong independent predictor of a 1-year mortality (OR 2.75; p<0.001).31 Other variables associated with higher rates of CI-AKI in diabetic patents are hypercholesterolaemia, contrast volume used >80 ml and insulin therapy.32
Conditions such as severe hypotension and CHF, particularly if requiring pressors or an intra-aortic balloon pump, can activate mechanisms of water reabsorption and concentrate CM in the tubules.25 In addition, activation of the renin-angiotensin-aldosterone axis causes vasoconstriction of kidney vessels that contributes to the ischaemic injury on the renal parenchyma. Similarly, anaemia generates a hyperdynamic circulation with peripheral vasoconstriction and higher risk of peripheral hypoxia.33 In animal models with CKD, anaemia seemed to promote damage of the renal proximal convoluted tubules and to reduce erythropoietin response to ischaemia. In vitro and in vivo studies have suggested erythropoietin might have proangiogenic and anti-apoptotic effects on endothelial cells and promote renal functional recovery in models of hypoxic and ischaemic renal injury.33 In the presence of CKD and anaemia this protective mechanism might be impaired. In the clinical practice, it has been observed that a decrease in haematocrit of >6 % places patients, usually women, almost at double the risk of developing CI-AKI.
Nephrotoxic drugs such as aminoglycosides, cyclosporin A, amphotericin, cisplatin and nonsteroidal anti-inflammatory drugs, undoubtedly favour the onset of CI-AKI. In addition, angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists can be a risk factor for CI-AKI.34 These drugs are very common among cardiac patients undergoing PCI, but when the GFR >60 ml/min/1.73 m2 their use rarely results in clinically relevant kidney damage upon exposure to CM. Conversely, in patients with CKD the synergistic effect of chronic use of ACE inhibitors and CM administration increases the risk of CI-AKI35 and might accelerate CKD progression.
Patients’ age is strongly associated with the risk of CI-AKI. It is well known that with aging, the number of functioning nephrons in the kidney progressively decreases. In addition, older patients will frequently present most of the fixed risk factors described such as CKD, CHF, diabetes and hypertension. When analysed per age quartile and sex, the incidence of CI-AKI was similar between men and women in the youngest age cohorts. However, women had higher prevalence of CI-AKI compared with men in the 65- to 79-year-old (14.5 % versus 11.0 %; p<0.001) and >80-year-old (18.7 % versus 15.1 %; P=0.048) age groups. Adding to the age-related risk of CI-AKI, women usually have higher rates of anemia and CKD.36 According to Chen et al., stage 3 CKD is present in 74 % of women and 45 % of men at time of admission for angiography.37
Preventive Measures
Currently there is no specific treatment for CI-AKI once the injury has occurred, therefore it is critical to stratify the risk and implement all measures to prevent CI-AKI in selected patients. The main prophylactic strategies comprise: reduction of modifiable risk factors (anaemia, hypotension, use of nephrotoxic drugs), reduction of CM exposure and peri-procedural oral or intravenous hydration (see Figure 1). Additionally, treatment with acetylcysteine, ascorbic acid and statins has been evaluated over the years with discordant results.
Reduction of Contrast Media Volume and Contrast Selection
Risk of CI-AKI depends directly on the volume of CM injected during the procedure and further increases in case of multiple staged interventions within 72 hours.38 Intra-arterial injection of CM, especially close to the renal arteries, is more frequently associated with CI-AKI than venous injection, probably because of the volumes used and the higher acute intrarenal concentrations. In the setting of primary PCI when few clinical and laboratory data are available before the procedure the ratio of contrast media volume (CMV) used to the estimated creatinine clearance (CMV/CrC) has been validated as an independent predictor of CI-AKI. The cut-off value varies depending on the study. Lanky et al. have reported a cut-off for CMV/CrC of 3.7,39 while Oreto et al. suggested a cut-off of 2.5.40 Gurm et al. observed an increased risk of CI-AKI and nephropathy requiring dialysis when CMV/CrC exceeded 2 and the risk became strongly significant when the ratio reached 3. As a general rule CM volume should be restricted accordingly to twice or thrice the eGFR.
In patients with significant renal impairment (stage 3 CKD or more), 30 ml of contrast for diagnostic catheterisation, and 100 ml in case of PCI would be a reasonable target. In all patients, the maximal acceptable contrast dose (MACD), defined as 5 ml × body weight (kg)/ baseline serum creatinine (mg/dl) should not be exceeded.41 The introduction of automated injection devices that allow the operator to set a maximum dose of CM delivered with any injection has greatly reduced the total volume of contrast used during coronary angiography and PCI.42 In addition, expansion of new imaging modalities could be used to minimise the need for angiographic images and therefore reduce the CMV injected. Optical coherence tomography (OCT) has been reported as a useful tool to guide PCI. However, since CM injection is required during each acquisition, despite its advantages for vessel visualisation, OCT use might not result in a significant reduction of CM. Recently, the Minimizing cOntrast utiliZation With IVUS Guidance in coRonary angioplasTy (MOZART) trial has tested the use of IVUS to guide PCI and reduce CMV exposure. This study randomised 83 patients to angiography-guided PCI or IVUS guided PCI. For patients in the latter group, operators were encouraged to use IVUS to the limit of its potential in order to minimise acquisition of angiographic images. Results showed that use of IVUS to guide PCI is safe and significantly reduces the CMV used up to 3 folds compared to controls. Although the trial was not powered to detect changes in post-PCI renal function, a trend towards reduction of CI-AKI events was observed.43
Selection of the type of CM is also very important. Most hyperosmolar CM have demonstrated similar dose-dependent pro-apoptotic effects on endothelial cells in vitro and are currently now no longer used in most countries. Conversely, low-osmolar contrast media (LOCM) and iso-osmolar contrast media (IOCM) are less nephrotoxic and are strongly recommended in clinical practice guidelines especially for patients with prior renal dysfunction.22,44 A recent meta-analysis by Dong et al. including 3,129 patients, showed that the IOCM iodixanol significantly decreased the risk of CI-AKI compared with a pool of LOCM (iopromide, iopamidol, iohexol, ioversol, ioxaglate and iomeprol) when CM was injected intra-arterially.45 Conversely, a large meta-analysis including 36 randomised controlled trials did not find a statistically significant reduction in biochemical CI-AKI for IOCM compared with all LOCM agents (pooled OR 0.77; 95 % CI; [0.56–1.06] P=0.11). However, a benefit in terms of reduced CI-AKI incidence was found when comparing the IOCM iodixanol to one specific LOCM, iohexol (pooled OR 0.25; 95 % CI; [0.11–0.55]).46 Importantly, in the Ionic versus non-ionic Contrast to Obviate worsening Nephropathy after angioplasty in chronic renal failure patients (ICON) trial, when tested against another LOCM, iodixanol did not reduce renal deterioration in patients with preexisting renal impairment.47
Peri-procedural Hydration
Peri-procedural administration of intravenous fluids remains the cornerstone treatment for the prevention of CI-AKI in all patients.48 Volume expansion increases urine filtration rate and reduces the concentration of CM in the tubular fluid. The timing, rate and duration of intravenous fluid administration for the prevention of CI-AKI is unclear.49 Operating centres often implement different protocols based on empirical experience. Current clinical practice guidelines and consensus statements recommend intravenous (IV) hydration with isotonic 0.9 % NaCl 1.0–1.5 ml/kg/h started 3–12 hours before the procedure and continued for 12–24 hours after the exposure to CM. In case of a same-day procedure, a faster hydration with 3 ml/kg/h can be used at least 1–3 hours before and 6 hours after the procedure.50,51 Recently, results from the Prevention of Contrast Renal Injury with Different Hydration Strategies (POSEIDON) trial showed that fluid administration guided by end diastolic left ventricular pressure seemed safe and effective.52 All patients in the POSEIDON trial received a bolus infusion at 3 ml/kg of 0.9 % NaCl for 1 hour before the procedure. Additionally, patients randomised to Left ventricular end-diastolic pressure (LVEDP)-guided hydration received 5 ml/kg/h, 3 ml/kg/h or 1.5 ml/kg/h of 0.9 % NaCl for LVEDP of <13, 13–18 mmHg, and >18 mmHg respectively, for 4 h after the procedure. All patients in the control group were hydrated at 1.5 ml/kg/h with 0.9 % NaCl for the same amount of time. The rate of CI-AKI was significantly lower in patients with LVEDP-guided hydration compared to control (6.7 % [12/178] versus 16.3 % [28/172]; relative risk 0.41; 95 % CI [0.22–0.79]; P=0.005). At 30 days and 6 months after the index procedure, incidence of CI-AKI, all-cause mortality, myocardial infarction, or renal replacement therapy was significantly lower in the LVEDP-guided hydration group.53 In 2004, the use of isotonic sodium bicarbonate, instead of saline was reported to be associated with a reduced incidence of acute CI-AKI. This result was attributed to the alkalisation of the renal parenchyma with a potential reduction in ROS production. However, subsequent studies have failed to confirm the benefit of sodium bicarbonate over isotonic saline.53,54
N-acetylcysteine and Ascorbic Acid
Since production of reactive oxygen species plays an important role in CI-AKI, use of antioxidant agents has been evaluated over the years. N-acetylcysteine (NAC) is a potent scavenger of ROS and improves endothelium-dependent vasodilation. In animal models of ischaemia-reperfusion injury, NAC significantly reduced kidney damage.55 However, contrasting results have been obtained in clinical studies testing NAC for the prevention of CI-AKI. The reason for this can probably be attributed to differences in the study population, the study design and the cut-off values used to determine CI-AKI. Importantly, NAC has poor bioavailability, around 10–20 %, due to first pass effect and has a half- life of about 5 hours.55 Therefore, early administration before the procedure is unlikely to result in a clinical benefit. In addition, daily drug dose and route of administration might explain the different results. Carbonell et al. have seen a beneficial effect with an intravenous dose of 600 mg twice a day whereas other studies using up to 2400 mg of oral NAC did not significantly reduce the rate of CI-AKI.56
However, a recent meta-analysis comprising 1916 patients treated with intravenous NAC failed to prove a clinical benefit of NAC use for the prevention of CI-AKI.57,58 Results from the recently started Prevention of Serious Adverse Events Following Angiography (PRESERVE) trial might provide further information on the use of NAC. This randomised double blind study will comprise 8,680 high risk patients undergoing angiography and it will test the efficacy of NAC versus placebo and sodium bicarbonate versus 0.9 % NaCl hydration.58 In the meantime, the use of NAC for the prevention of CI-AKI is not recommended in the 2011 AHA/ACC guidelines for PCI.50 However, since NAC is inexpensive and appears to be safe at the doses used for CI-AKI prevention, its use could be considered in intermediate-high risk patients despite the inconclusive evidence available. The 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines on acute kidney injury support the use of oral NAC, in combination with intravenous crystalloids in patients at risk for CI-AKI.59
Ascorbic acid has also been used in randomised clinical trials because of its antioxidant properties. In vitro and animal studies suggest that ascorbic acid may augment NO supply and reduce the oxidative stress in the renal tubules and peritubular capillaries thus reducing the extent of CI-AKI.60,61 Similar to NAC results on the efficacy of ascorbic acid in this setting have been discordant.61,62 Nevertheless, a meta-analysis by Sadat et al., analysing data from nine randomised clinical trials for a total of 1,536 subjects, showed that patients with preexisting CKD receiving peri-procedural ascorbic acid either intravenously or orally had a lower prevalence of CI-AKI compared to those treated with placebo.63 However, so far ascorbic acid is not mentioned as a preventive measure in the current KDIGO clinical practice guidelines.59
Statins
Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are known to exert pleiotropic effects beyond cholesterol lowering. In particular, in vitro studies suggest statins have anti-inflammatory and antioxidant properties that could reduce production of ROS and avoid cell apoptosis after CM exposure. When tested in patients, a single high loading dose of atorvastatin 24 hours before CM exposure was sufficient to reduce the rate of CI-AKI in patients with diabetes or moderate CKD.64 Conversely, the Prevention of Radiocontrast-Medium-induced nephropathy using short term highdose simvastatin (PROMISS) trial, evaluating simvastatin use in patients with CKD, failed to prove an effect on the rate of CI-AKI.65 Subsequent studies, mostly testing different regimens of rosuvastatin treatment, obtained positive results. For instance, the Protective Effect of Rosuvastatin and Antiplatelet Therapy On contrast-induced acute kidney injury and myocardial damage in patients with Acute Coronary Syndrome (PRATO-ACS) trial has shown that high doses of rosuvastatin (40 mg loading dose + 20 mg maintenance dose) in patients undergoing PCI significantly reduced CI-AKI and the 30-day rate of cardiovascular and renal events comprising persistent renal damage and dialysis.66 Rosuvastatin seemed effective even when used for a short time.
Zahng et al. described a reduction of CI-AKI and a composite of death, dialysis and worsened heart failure at 30 days in patients treated with peri-procedural rosuvastatin consisting of 10 mg daily starting 2 days before PCI and continued for 3 days after the procedure.67 Overall, recent meta-analyses support the use of statins for the prevention of CI-AKI.68–70 Current clinical practice guidelines do not yet reflect the recent results on the use of statins for CI-AKI. However, while AHA/ACC guidelines on the management of non ST-segment elevation myocardial infarction (NSTEMI) suggest starting statin treatment before hospital discharge, based on recent evidence from randomised clinical trials and meta-analysis, the use of peri-procedural statins seems reasonable in patients undergoing PCI especially if presenting with risk factors for CI-AKI.
Fenoldopam Mesylate
Fenoldopam mesylate is a selective dopamine-1 receptor agonist that induces systemic and regional vasodilation. Because of its quick mechanism of action, intravenous fenoldopam is approved by the US Food and Drug Administration for the treatment of urgent and emergent hypertension. In 2002, Tumulin et al. observed that fenoldopam prevented contrast-induced vasoconstriction of renal vessels thus significantly increasing renal blood flow. When tested on patients with CKD, intravenous fenoldopam resulted in a lower rate of CI-AKI compared to saline.71 Subsequently, a meta-analysis comprising 16 clinical trials for a total of over 1,200 patients showed a significant reduction of CI-AKI (OR 0.43; 95 % CI; [0.32–0.59]; P<0.001), need for renal replacement therapy (OR 0.54; 95 % CI; [0.34–0.84]; P=0.007), and in-hospital death (OR 0.64; 95 % CI; [0.45–0.91]; P=0.01)72 with fenoldopam. Despite these promising results, larger randomised clinical trials on patients with CKD and CM exposure have failed to find any significant clinical benefit of fenoldopam use.73,74 Therefore, current KDIGO guidelines do not recommend the use of fenoldopam for the prevention of CI-AKI.59
Theophylline
Since an increase in serum and urinary adenosine has been observed after CM administration in CI-AKI patients, treatment with theophylline, a non-specific adenosine receptor antagonist, has been evaluated. Although a mild nephroprotective effect has been observed in a few clinical trials, the potential cardiovascular side effects and its interactions with numerous drugs currently limit the use of theophylline in the clinical practice.75
Renal Replacement Therapy
Renal replacement therapies (RRT) with haemodialysis (HD) or continuous renal therapy (CRRT) have been evaluated as periprocedural prophylactic measures to prevent CI-AKI in patients at risk. However, clinical studies have found no benefit of RRT for the prevention of CI-AKI in patients with stage 3 CKD.76 Nevertheless, RRT with HD or CRRT could be considered only for patients with stage 4 or 5 CKD who are not on periodic haemodialysis. Approximately 3 % of patients with CKD develop severe AKI requiring dialysis. In these patients, RRT could reduce in hospital stay length and mortality.77
Current Clinical Practice Guidelines
KDIGO are international evidence-based clinical practice guidelines published in 2012 that comprise current recommendations on the prevention and management of AKI.59 These guidelines include a comprehensive section on CI-AKI that gathers, expands and updates indications on this subject, previously available in interventional cardiology guidelines on the management of coronary artery disease. Acceptable definitions of CI-AKI have been expanded in the 2012 KDIGO document and now comprise an increase in serum creatinine (SCr) by ≥0.3 mg/dl (≥26.5 μmol/l) within 48 hours or an increase in SCr to ≥1.5 times baseline within 7 days, or a urine volume <0.5 ml/ kg/hour for 6 hours after contrast exposure. Importantly, the cut-off values for SCr and eGRF to identify patients at risk of CI-AKI are a baseline SCr concentration ≥1.3 mg/dl (≥115 μmol/l) in men and ≥1.0 mg/dl (≥88.4 μmol/l) in women, equivalent to an eGFR of <60 ml/ min/1.73 m2. Consistent with AHA/ACC guidelines on percutaneous coronary interventions,50 reduction of the CM volume used and selection of either iso-osmolar or low-osmolar iodinated contrast media are strongly advised. Among the preventive measures listed in this review, KDIGO suggests the use of oral NAC, together with intravenous isotonic crystalloids, in patients at increased risk of CI-AKI. However, as previously mentioned, use of theophylline, fenoldopam and prophylactic IHD or haemofiltration are not recommended for the prevention of CI-AKI.
Finally, although discussed in the KDIGO guidelines, there is no explicit recommendation regarding the use of ascorbic acid and statins.59 While evidence on ascorbic acid is still controversial, we believe it would be reasonable and safe to adopt a peri-procedural treatment strategy with statins for the prevention of CI-AKI in selected patients at risk based on the promising results of recent randomised clinical trials.
Conclusion
CI-AKI in patients with preexisting renal damage or risk factors for the development of kidney dysfunction is a potentially serious complication after angiographic procedures with increased short and long-term morbidity and mortality. Appropriate management of patients at risk is crucial for the prevention of CI-AKI. Although a specific treatment for CI-AKI is not available removal of modifiable risk factors and implementation of periprocedural measures such as CM reduction and intravenous hydration can significantly lower the risk of CI-AKI in selected patients.