Aortic stenosis (AS) is the most common valvular heart disease in industrialised countries with a prevalence of about 5 % in the general population aged greater than 75 years. During the past decade, transcatheter aortic valve replacement (TAVR) has emerged as a valuable, minimally invasive treatment option for patients presenting with symptomatic severe AS, who due to their advanced age and relevant comorbidities are at prohibitive risk for conventional surgery,1 whereas surgical aortic valve replacement (SAVR) remains the gold standard for the treatment of symptomatic AS in patients with low to moderate surgical risk.2 However, many patients begin to experience AS-related symptoms late in their lives when multiple comorbidities preclude surgery as an option. As a result, before the advent of TAVR, patients considered high- or extreme-risk surgical candidates were once limited to conventional medical therapy. Ever since the first device was deployed in 2002, TAVR has enabled inoperable patients the opportunity to experience survival rates equivalent to their surgical counterparts with considerably less procedural risk.1 Therefore, the number of patients undergoing TAVR has increased steadily, and the complications related to valve implantation have been well defined.
The development of atrioventricular (AV) conduction disturbances is one of the most commonly encountered complications associated with TAVR. Between 3 and 6 % of patients undergoing surgical replacement of their aortic valve will develop complete heart block (CHB),3 while considerably higher rates have been reported in the setting of TAVR in individual studies.4 In this review, we aim to explore the significance of conduction disturbances preceding and resulting from TAVR. We have focused on data that raise concerns around creating chronic left ventricular (LV) dyssynchrony in this patient population, either as a consequence of creating left bundle branch block (LBBB), or from chronic right ventricular (RV) pacing. Additionally, we reviewed a number of factors that predispose TAVR patients to develop conduction disturbances, and clinical factors that can be used to identify those patients likely to require permanent pacing and alternatively those in whom it may be worth waiting longer prior to committing to permanent pacemaker (PPM) implantation. Shy of unique valve designs, it has become clear there are only modest improvements an operator can make to avoid the complication of heart block and this complication is simply part of the procedure. It is critical to anticipate it and think prospectively about the ideal pacing mode for the individual patient to prevent chronic LV dyssynchrony in those patients at highest risk.
Mechanisms of Heart Block
Anatomical Relationship of the Cardiac Conduction System and the Aortic Root
Since the 16th century when Leonardo da Vinci conducted the first known cadaveric studies of the heart, the aortic root complex has been studied extensively. With the advent of percutaneous valves, there has been renewed interest in understanding the anatomy of the aortic valve, particularly with respect to the conduction system since the proximity of the latter to the aortic root can result in its disruption during valve deployment.
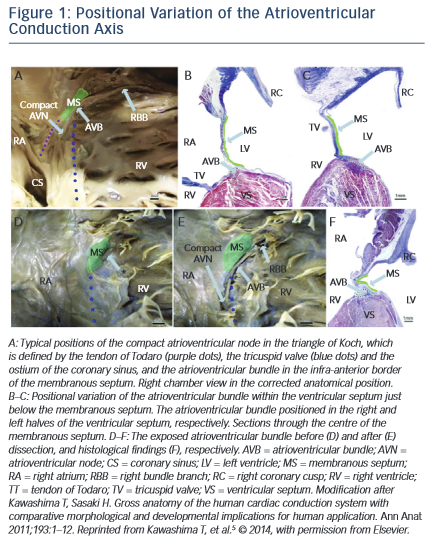
Within the right atrium, the AV node is positioned at the base of the atrial septum and is located using landmarks that form the triangle of Koch – the tendon of Todaro, the orifice of the coronary sinus and the insertion point of the tricuspid valve septal leaflet. The antero- posterior relationship of the AV node with respect to the apex of the triangle of Koch varies between individuals as does the length of the non-penetrating (or most proximal) portion of the His bundle. The non-penetrating portion of the His bundle traverses the membranous septum to become the penetrating His bundle, which then physically divides into the respective bundle branches. Inter-individual variation in the penetrating bundle length and depth of septal penetration and variation in the location of the proximal portion of left bundle determine how susceptible these structures are to injury during TAVR. These anatomical variations have been elegantly characterised by Kawashima and Sato.5 Specifically, they describe three major variants in these anatomical relationships that, depending on which is present, determine the susceptibility of a patient to developing complete or LBBB. In an autopsy series of 115 elderly patients, 50 % were found to have a relatively right-sided AV bundle, 30 % a left-sided AV bundle, and in around 20 % the bundle coursed under the membranous septum just below the endocardium. In the latter two variants, the AV bundle is particularly exposed and susceptible to injury. LBBB susceptibility is determined by how soon the left bundle appears on the left side of the septum, and injury to both is further affected by the relative positioning of the membranous septum with respect to the aortic cusps, see Figure 1.
Mechanical Effects of Bioprosthetic Valves on the Conduction System
Currently, two TAVR devices are in clinical use – the self-expanding CoreValve (Medtronic) and the balloon-expandable SAPIEN valve (Edwards). A network meta-analysis of randomised trials (n=1,805 patients) comparing TAVR (using CoreValve or SAPIEN valve) versus SAVR showed similar survival rates at a median of 8 months, but revealed higher rates of PPM implantation using the transcatheter approach compared with surgical AVR.6 Conduction abnormalities from SAVR are attributed to the surgical method – suturing along the sewing ring near the membranous septum, removal of the native aortic valve and the resultant oedema. TAVR obviates some of these concerns, but raises with it a slew of device-specific issues including differences in size and shape, deployment method and vascular access site.
The susceptibility to AV block in the TAVR setting is device specific, as has been well-described in meta-analyses, with incidence ranging between 24.5 % and 25.8 % in the CoreValve device compared with 5.9 % to 6.5 % in the SAPIEN valve.7 The increased risk of AV block with the CoreValve has been attributed partly to the valve design (self-expanding versus balloon-expandable) and the potential for a deeper valve implantation into the left ventricular outflow tract (LVOT). The aforementioned mechanism may result in more injury to the AV node and left bundle branches, which may be delayed because of the self-expanding nature of the prosthesis and tissue oedema.3,8,9 A Spanish study (n=65; CoreValve only) reported a frame depth in the LVOT of 11.1 mm as an independent predictor of PPM insertion with 81 % sensitivity and 84.6 % specificity.10 Furthermore, the self-expanding property of the CoreValve stent is thought to impart a significant degree of persistent radial force to the aortic annulus, particularly at the level of the frame skirt, which is adjacent to the LVOT and may contribute to a higher incidence of post-implant heart block.11 In contrast, the balloon-expandable bovine SAPIENvalvehasasmallerprofile(14–19mminlength)andiscomposedof either stainless steel or cobalt-chromium. SAPIEN valve has a cylindrical shape and is associated with much lower need for permanent pacing. Finally, the prosthesis:LVOT diameter ratio was recently identified as a novel predictor for permanent pacemaker implantation even among patients undergoing TAVR with SAPIEN valve (for each 0.1 increment, OR 1.29; 95 % CI [1.10–1.51]; p=0.002).7 Hence pre-operative evaluation is probably relevant in mitigating the probability of provoking heart block in terms of both device selection and sizing.
Transfemoral Versus Apical Access
Whether or not TAVR is performed via the transfemoral (TF) or transapical (TA) approach does not seem to significantly impact pacemaker implant incidence. A recent single-centre retrospective study from the US comparing TF versus TA access (n=123 high-risk AS patients) found no significant differences with respect to 30-day mortality, MI or stroke; the same was true for the secondary endpoint of pacemaker insertion (TF 9.1 % vs TA 12.3 %; p=0.574).12
Clinical Predictors of AV Block
It is easy to generalise conduction abnormalities to mechanical factors alone, but the involved patient population has multiple comorbidities that are probably relevant to this issue. As with any operation, procedural complications increase with a patient’s age and comorbidities. In severe aortic stenosis, common cardiac risk factors including diabetes mellitus, hypertension and congestive heart failure have been associated with the development of LBBB and bradyarrhythmias, irrespective of whether or not these patients underwent surgical or transcatheter AVR.13
Indications for Permanent Pacemakers
Pacemaker indications following TAVR encompass a gamut of disease states including sinus node dysfunction, atrial fibrillation with slow ventricular response and symptomatic bradycardia.14 The PARTNER registry showed, however, that 80 % of single- or dual-chambered pacemakers were implanted for high-degree or CHB.4 Physiologically, this can be explained, as new-onset LBBB is the most common conduction disturbance after TAVR. In patients with pre-existing right bundle branch block (RBBB), CHB will inevitably occur, and expert consensus recommends placing a temporary transvenous pacemaker not only for patients with complete AV dissociation, but for those at risk for heart block.
The question then becomes quite challenging, as physicians have to decide, first, which patients with AV block are candidates for permanent pacing; and, second, just when a pacemaker should be implanted. To answer these questions, case series involving CoreValve were performed and revealed a surfeit of factors predictive of PPMs following TAVR, including left axis deviation, mitral annular calcification and narrow LVOT diameter, to name just a few.3,15 Admittedly, these case series sampled a small number of patients and were mainly hypothesis generating until larger registries could be examined.
Device-specific (CoreValve versus SAPIEN valve) Clinical Predictors
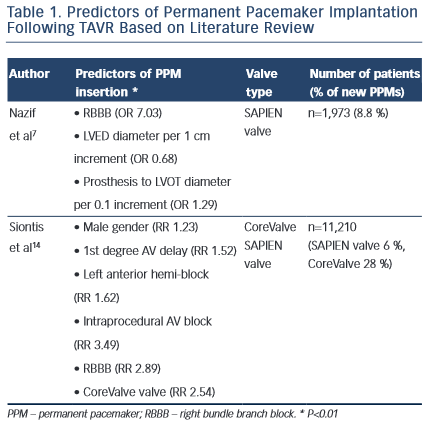
In 2014, a meta-analysis across 41 studies (n=11,210 TAVR patients) revealed a 17 % incidence of PPMs. The aim of this study was to identify clinical determinants predictive of PPM implantation. Both CoreValve and SAPIEN valve were included in the study. Crude relative risks of 14 eligible variables were examined and the following were identified as predictors of PPM implantation following TAVR: male gender (RR 1.23; p< 0.01), first-degree AV delay (RR 1.52; p<0.01), left anterior hemi-block (RR 1.62; p< 0.01), RBBB (RR 2.89; p<0.01) and intraprocedural AV block (RR 3.49; p< 0.01). As stated, data on the SAPIEN valve were limited and these clinical variables were applied mainly to patients that received the CoreValve bioprosthesis.4
The PARTNER registry houses the largest database of SAPIEN valve patients and a recent subset analysis outlined predictors of permanent pacemakers specifically for the SAPIEN device. Of 1,973 SAPIEN valve cases, PPM was required in 8.8 % of patients. Multivariable analysis revealed baseline RBBB (OR 7.03; 95 % CI [4.92–10.06]; p=0.002), LVED diameter (for each 1 cm, OR 0.68; 95 % CI [0.53–0.87]; p=0.003) and prosthesis to LVOT diameter (mentioned earlier) as significant predictors. Furthermore, patients who received PPMs had higher rates of mortality and repeat hospitalisation at one year (42 % vs 32.6 %; p=0.007).7
Extrapolating the results across multiple studies, baseline RBBB is a consistent predictor of PPM insertion irrespective of the device used (CoreValve versus SAPIEN valve). As previously discussed, new LBBB manifests frequently after TAVR, reported to appear in up to 65 % of patients, and when compounded with RBBB it will lead to CHB. Other determinants of PPM implant, such as prosthesis to LVOT diameter, point toward the mechanical effects of conduction abnormalities, highlighting the close proximity of the aortic annulus to the AV bundle and its branches, Table 1.
Timing of PPM Implant
The challenge with peri-procedural heart block is determining when to implant a PPM. Guidelines related to timing do not exist and for obvious reasons AV block after TAVR exhibits dynamic properties. Nazif and colleagues reported resolution of LBBB in approximately 40 % of SAPIEN valve patients just one month following TAVR, consistent with previous studies that demonstrated ranges between 30–50 %7,16. Furthermore, a single-centre prospective study demonstrated recovery of native conduction among patients with PPMs through interrogation of their devices at 1 to 40 months’ follow-up. Of the 167 patients with PPMs after TAVR, only 44 % were pacemaker dependent, defined by the persistence of high-degree AVB or absence of ventricular escape at VVI 30 bpm.17 These findings propose an important paradigm shift focusing less on who will develop, but rather who will remain in heart block.
Recently, a new wrinkle has been added to the discussion of peri- procedural heart block. Urena and colleagues performed a prospective study of 435 patients and identified new arrhythmias in 16.1 % of patients during the 1 day preceding TAVR. The arrhythmias discovered by 24-hour continuous monitoring included paroxysmal atrial fibrillation, advanced AV block, severe bradycardia and nonsustained ventricular tachycardia, accounting for one-third of post-procedural conduction abnormalities. Since there was no control group in this study, it is unclear if the arrhythmias found on continuous monitoring were attributable to severe aortic stenosis. Nevertheless, these findings pose the possibility that what we deem as novel arrhythmias following TAVR may not be new. More importantly, if these findings by Urena are validated in future studies, management of TAVR patients would change, as pre-procedural arrhythmias were associated with higher cerebrovascular events.18
As of this writing, most patients will receive a PPM within 1 week of TAVR for high-degree or CHB.19 However, careful review of PPM indications from procedure reports from the PARTNER registry revealed a diagnosis of sick-sinus syndrome in 17 % of patients, higher than found in previously published reports. Additionally, there was a noticeable difference in PPM rates between the continued access registry and randomised trial (9.6 % versus 5.6 %), indicating that PPM insertion is partly driven by variable physician threshold.7
Sequelae of PPM
Significance of LBBB
We touched upon the mechanism and rates of new LBBB in previous sections and now shift our focus to its prognostic significance. Overall, mortality data in patients who develop new LBBB after TAVR are conflicted. The largest study published to date (n=1151) showed no association between new LBBB and death, but led to an increase in pacemaker insertion and failure of LVEF to improve at 1-year follow- up.20 In contrast, a Dutch registry (n=679) followed patients for a median of 450 days and showed that new LBBB was an independent predictor of all-cause mortality (37.8 % vs 24 % [no LBBB]; p=0.002). The authors postulate that LBBB-induced dyssynchrony and progression to higher degree heart block are two possible mechanisms behind the higher mortality rates.21 Smaller case series have demonstrated an increase in syncopal events and PPM insertion in patients with persistent LBBB and may be related to the progression of LBBB toward CHB described above.22,23
Previously, TAVR has been shown to improve or maintain left ventricular function (LVF), especially among patients with baseline depressed ejection fraction.24 In those with persistent LBBB, however, there is greater propensity for LV dyssynchrony and failure of LV ejection fraction to recover at one year.20 The effect that LBBB has on LV function may be akin to right ventricular (RV) pacing, which results in a similar pattern of electrical propagation. In both instances, the right ventricle is activated first and then crosses the interventricular septum via cell-to-cell conduction, bypassing physiological activation by the native His-Purkinje system. As a result of asynchronous conduction, abnormal septal wall motion and alterations in regional blood flow in the LV myocardium have been demonstrated.25
Clinically, several trials have highlighted the detrimental effects of long-term RV pacing on LV function. MOST (Mode Selection Trial) identified a significant correlation between frequency of RV pacing with the development of heart failure, noting that the lowest risk patients had a ventricular pacing burden <10 % (DDDR mode).26 These findings were validated by MADIT II (Multicentre Automatic Defibrillator Implantation Trial), which examined patients with prior MI (LVEF less than or equal to 30 %) who were randomised to receive an implantable cardiac defibrillator (ICD) or no device. Patients in the ICD group with high ventricular pacing burden had a significant rate of heart failure.27 Additionally, patients in the DAVID (Dual Chamber and VVI Implantable Defibrillator) trial who were continuously paced from the RV (DDDR with lower limit of 70 bpm) had a hazard ratio of 1.61 (95 % CI [1.06– 2.44]; p=0.03) with respect to the composite end-point of heart failure and death when compared with patients whose devices were set at a backup rate of VVIR 40 bpm.28 It remains to be seen if new, persistent LBBB after TAVR will duplicate these findings, as long-term follow-up data have yet to be collected, but the totality of current data support the idea that these concerns should be incorporated into current clinical practice in the post-TAVR population.
Pacing Modes
The optimal pacing strategy is one that limits RV pacing and preserves native AV conduction. Novel algorithms have been developed to achieve these effects. AV search hysteresis (AVSH) is a feature in dual chamber (DDD) devices that enables temporary lengthening of the preset AV delay to see if native conduction occurs. If there is conduction with a long PR interval, AVSH will stretch the preset AV delay to the degree necessary to ensure physiologic conduction. There is a limit to the extent at which AV intervals can be set, so that AF detection and upper rate behavior in DDD devices can be optimised.
A different approach that is also employed is to uncouple atrial activity altogether from ventricular pacing. This algorithm defaults DDD devices to AAI/R mode and the ventricle is monitored on a beat-to-beat basis, switching to DDD/R mode if loss of AV conduction is detected (defined as two out of four A–A intervals missing a ventricular event). This algorithm will periodically check for intrinsic AV conduction, which if present, will revert back to AAI/R mode with ventricular monitoring. A crossover multicentre randomised trial compared this approach with standard DDD/R ICDs and found that the former resulted in significantly lower burden of RV pacing (4.1 +/- 16.3 vs 73.8 +/- 32.5, p<0.0001).29
Future considerations
TAVR is a promising treatment strategy, which is likely to be expanded beyond its current indication for high- and extreme-risk surgical patients in the near future. SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation) is an ongoing trial randomising patients with intermediate operative risk to CoreValve versus SAVR.30 Moreover, in March 2015, the Food and Drug Administration (FDA) approved CoreValve for the treatment of bioprosthetic aortic valve failure via a valve-in-valve approach.31 With the expected increase in procedural volume, the concerns regarding management of conduction disorders will also increase.
New iterations of CoreValve and SAPIEN valve continue to evolve, with novel designs already at varying stages of development. In addition, new generation TAVR systems have been designed with the ability to be retrieved and repositioned or having features that facilitate optimal device placement. These devices, all recently CE mark approved and currently being studied, include the Lotus valve (Boston Scientific), the Engager (Medtronic), the JenaValve (JenaValve Technology), the Acurate (Symetis), the Direct Flow Medical valve (Direct Flow Medical), and the Portico (St Jude Medical). Each of these releases will present a set of unique challenges, with varying effects on conduction disorder probabilities.
In the case of the latest SAPIEN valve (SAPIEN 3; Edwards), a retrospective study (n=125) showed an impressive reduction in significant paravalvular regurgitation, but observed a PPM rate of 25.5 %, a similar rate to CoreValve.32 However, a study comparing the outcomes of TAVR with the Lotus valve versus the SAPIEN 3 valve showed a significantly higher need for pacemaker implantation in the former (27 % vs 4 %; p<0.003).33 The JenaValve device and Acurate valve also demonstrated relatively low pacemaker implantation rates (9.1 % and 10.0 %), though in a small prospective trial and in a short-term registry study.34,35 While the reduced profile and improved features of novel valve designs strive to minimise post-procedural conductional abnormalities, further study with larger datasets will be needed to determine if this is in fact the case.
Conclusion
The emergence of TAVR has provided inoperable patients with severe aortic stenosis a viable treatment option with meaningful survival benefit over medical therapy alone. However, the benefit of this procedure comes at the cost of a substantially increased risk of high- grade heart block that necessitates the placement of a permanent pacemaker. As the popularity of TAVR grows and the indications for its use widen, it has become increasingly important to identify patients at risk for the development of conduction abnormalities. A number of mechanical and clinical features have been implicated as risk factors for PPM placement after TAVR, including usage of the larger profile CoreValve device, deeper implantation within the LVOT (CoreValve), prosthesis to LVOT diameter ratio (SAPIEN valve), and pre-existing RBBB. These predictors are meaningful in this context, since it is well- established that the aortic root complex is intimately related to the AV node and its distal conduction fibres.
Additional questions exist regarding the optimal timing of pacemaker placement following TAVR. Recent evidence – albeit from a few small trials – has raised the possibility that a substantial number of patients who receive permanent pacemakers after TAVR are not pacemaker-dependent during follow-up visits. These data emphasise the dynamic nature of post-procedural heart block and suggest that PPM implantation is perhaps occurring too early in a subset of patients who will ultimately regain full function of their conduction system. Conceivably pre-procedure risk (i.e. the presence of RBBB) can be used to predict those patients likely to require permanent pacing, and close follow-up of the clinical progress of patients with persistent LBBB identify those patients likely to benefit from physiological pacing.
While the prognostic implications of new conduction abnormalities following TAVR remain somewhat nebulous, it is evident that these obstacles will continue to present challenges for physicians and patients in the foreseeable future. By improving our ability to predict conduction disturbances and our understanding of the mechanism by which this occurs, we can improve on valve design, decrease the number of unnecessary devices implanted, optimise pacing modes when pacing is unavoidable, and improve overall outcomes following TAVR.