Vasovagal syncope (VVS) is most common cause of syncope,1 with 37 % of the population suffering at least one attack during their life time.2 Head up tilt-table testing (HUTT) was first evaluated and used for the diagnosis of VVS in 1986.3–4 Prior to this, diagnosis relied purely on the clinical history and exclusion of other conditions. With almost 40 % of patients with VVS experiencing no prodrome5 prior to syncope, and the general acceptance that it was a condition of the young, VVS was significantly undiagnosed – particularly in the elderly.4,6–7 The exact mechanism of VVS is complex. It involves several autonomic reflexes, of which some are exaggerated (parasympathetic) and others depressed (sympathetic), resulting in a reduction in blood pressure and/or pulse rate and subsequent cerebral hypoperfusion and syncope.8–10 The mechanism can differ with subsequent attacks and from patient-to-patient. Most patients respond to the European Society of Cardiology (ESC) recommendation11 of lifestyle modification and withdrawal of vasoactive medications; however, a significant number of patients continue to have repeated syncopal and pre-syncopal attacks. There is no associated increase in mortality, so the condition is often referred to as benign. Despite this, in patients with refractory VVS the condition can be frightening, disabling and have significant impact on quality of life12 and there is an urgent need for an effective treatment option in these refractory patients. Permanent pacemakers13 and drug treatment, in particular beta-blockers14, selective serotonin reuptake inhibitors, fludrocortisone15 and midodrine,16 have not been proven or not adequately assessed to be beneficial in VVS and are therefore not routinely used.
Home orthostatic training (HOT) has been shown to improve autonomic measures after repeat assessments in non-age selected cohorts.17–19 However, HOT has not been tested in an exclusively elderly population. Therefore the aim of this study was to evaluate the effect of HOT on autonomic reflexes in elderly patients with VVS. Our research questions were:
- Are there different mechanisms of vasovagal syncope in elderly and young populations?
- What therapies are efficacious in vasovagal syncope?
- Are autonomic measurements useful in the assessment of vasovagal syncope?
Methods
Patients
Consecutive patients with positive HUTT were invited to participate in the study. All participants were aged >16 and had had at least two syncopal episodes in the previous 12 months and a positive HUTT. A positive HUTT was defined as a reduction in blood pressure associated with syncope. Exclusion criteria included the following: (i) inability to provide informed consent; (ii) physical or mental inability to perform HOT as determined by the investigators; and (iii) pregnancy.
Interventions
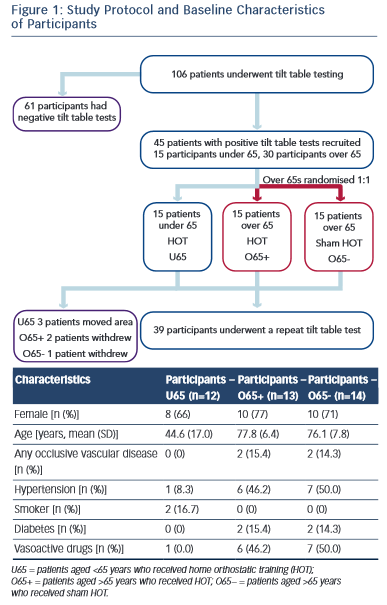
The local research ethics committee approved the study and written informed consent was obtained from all participants. All patients received normal clinical care in accordance with ESC guidelines for VVS. Subjects were divided into two cohorts: those aged >65 years (O65) and those aged <65 years (U65). The O65 group was randomised to receive either active HOT (O65+) or sham training (O65−). The U65 cohort were all actively trained and acted as an additional control group. Randomisation was performed by an independent clinician using computer-generated random numbers. Active or sham training was taught to the participant during a one-to-one training session. A written crib sheet of the training technique and regime was also provided. Participants and their clinicians not involved in the trial were blinded to the treatment group. Training was undertaken for a total of 3 months before a repeat HUTT was performed (Figure 1).
Active HOT
Participants in the U65 and O65+ groups were asked to stand with their backs against a wall supported by their head and shoulders and to move their feet 20 cm from the base of the wall. They were asked to hold this position for 30 minutes or until pre-syncopal symptoms occurred. Participants were warned to prepare the training area so that a prone position could be achieved quickly if symptoms occurred suddenly.
Sham Training
Participants in the O65− group were asked to stand with their backs against a wall supported by their head and shoulders but move their feet just 5 cm from the base of the wall. They were asked to hold this position for 5 minutes or until pre-syncopal symptoms occurred. As with the active group, participants were warned to prepare the training area so that a prone position could be achieved quickly if symptoms occurred suddenly.
Measurements
HUTT
A baseline and 3-month HUTT were performed. Participants were asked to fast for no longer than 2 hours prior to the test. All drugs affecting the validity of the HUTT were stopped at least five half-lives prior to testing. Participants were prepared with continuous ECG monitoring, a non-invasive blood pressure cuff, a digital non-invasive beat-to-beat blood pressure monitor and patches for impedance monitoring using the Task Force® Monitor system (CNSystems, Graz, Austria). The tilt-testing laboratory is temperature controlled, quiet and the test was performed under low lighting levels. Participants were laid in a supine position for 10 minutes to allow haemodynamic measures to stabilise before being tilted to 70°. This was maintained for 20 minutes, when 200 μg of sub-lingual glyceryl trinitrate was administered. A further 20 minutes of recording was performed. The test was terminated if syncope occurred. Time to syncope during the HUTT was recorded in seconds.
Heart Rate Variability (HRV)
The autoregressive method for HRV was used to calculate the low-frequency (LF) 0.04–0.15 Hz and high-frequency (HF) 0.15–0.4 Hz spectral densities from the continuous ECG recording for each HUTT phase. Automated software eliminated ectopic beats and artefactual recordings.
Baroreflex Sensitivity (BRS)
For each phase of HUTT the BRS was calculated using the sequence method. The slope regression was determined for increases in systolic blood pressure (SBP) accompanied by lengthening of the R-R interval (RRI) (up sequences) and decreases in SBP associated with shortening of the RRI (down sequences) for three or more consecutive R-waves. The blood pressure sequences were paired with the RRI at which the changes occurred.
Symptom and Training Diaries
Participants were asked to record their daily training regime and document reasons for failure or premature termination of training on each and every occasion. Any pre-syncopal symptoms or syncopal attacks were also documented.
Statistical Analysis
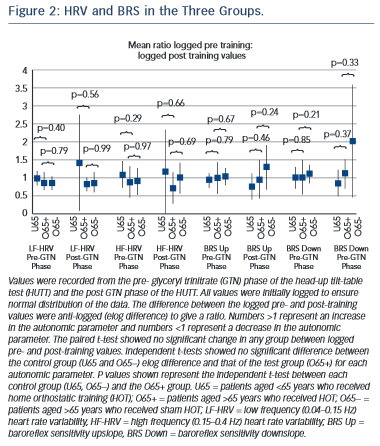
All continuous variables were reported as mean with SD for normally distributed data and median with interquartile range for non- normally distributed data. For the haemodynamic variables, LF-HRV, HF-HRV, upslope BRS, downslope BRS, the values were initially logged to ensure normal distribution. The paired student’s t-test was used for within-group comparisons of pre- and post-training logged haemodynamic data and time to syncope. For comparison of haemodynamic measures between groups, the log differences were calculated and then the elog differences were compared using the unpaired student’s t-test. Log ratios of pre- and post-haemodynamic values were recorded. A value of 1 indicated no change, <1 indicated a reduction in the haemodynamic measure and >1 indicated an improvement. A two-tailed P-value of <0.05 was considered statistically significant. PASW Statistics 18.0™ for Windows® was used for all data analysis.
Results
Overall, 106 participants with recurrent syncope underwent HUTT. Of these, 45 had a positive HUTT response and were diagnosed with VVS. The first 30 participants aged >65 years and the first 15 participants aged <65 years who met the study criteria were recruited.
Fifteen participants in the O65 group were randomised to active HOT. Two participants in the O65+ group declined the repeat tilt-table testing and their data were not used in the analysis. No reason was given. One participant in the O65− group suffered a hip fracture not related to the study. Three participants from the U65 group moved out of area and were lost to follow-up. Baseline characteristics were similar in the two O65 groups (Figure 1).
HRV
No significant difference was seen between pre- and post-training in any of the groups in any phase of HUTT (P=NS). Comparison of the elog difference of the pre- and post-training values for the U65 and O65− groups to the O65+ group showed no significant difference (see Figure 2).
BRS
No significant improvement from HOT was seen in any of the treatment groups or between any treatment group (Figure 2).
Time to syncope on HUTT
All except two participants (one in the O65+ group and one in the U65 group) had a positive HUTT on repeat testing. HOT did not significantly influence the time to syncope during HUTT (from 1473 seconds ± 336 to 1386 seconds ± 698 [P=0.67] in the U65 group; from 1574 seconds ± 278 to 1532 seconds ± 623 [P=0.80] in the O65+ group; and from 1454 seconds ± 332 to 1435 seconds ± 286 [P=0.84] in the O65− group).
Subjective Data
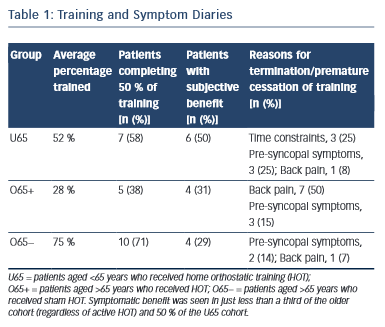
All participants in the analysis returned satisfactory training and symptom diaries, which were used in the subjective analysis. The most common cause of premature termination of training was back pain in the O65+ group (seven patients in the O65+ group [50 %], one patient in the O65− group [7 %] and one patient in the U65 group [8 %]). Time constraint was the reason for withdrawal in three patients in the U65 group (25 %) but none in either O65 group. Pre-syncopal symptoms were the reason for withdrawal in three patients in the O65+ group (15 %), two in the O65− group (14 %) and three in the U65 group (25 %). (Table 1). Comparison of the autonomic parameters pre- and post- training in those participants that experienced symptomatic benefit as opposed to those who did not revealed no significant differences.
Discussion
This prospective single-blind randomised trial is the first to assess HOT in an exclusively elderly population with VVS. Furthermore, with the exception of one study,19 all other randomised controlled trials20–21 examining the role of HOT have compared active orthostatic training to conventional therapy. The presence of the sham-training group in this study allows patient blinding to therapy and hence greatly strengthens the overall power. Unlike previous studies,19-20,22 no improvement in any autonomic cardiovascular reflex was observed in any of the study groups.
Early studies have assessed the changes in simple measures such as blood pressure, heart rate, time to first syncope and tilt-table result.17,21,23 These parameters have poor reproducibility24 and unless autonomic function is grossly altered, any subtle change will be lost in background noise. BRS and HRV spectral densities are being increasingly utilised as a sensitive research tool when looking at autonomic responses. The high sensitivity is in part because of the exponential changes in these values as opposed to linear changes in the traditionally-measured parameters. HRV spectral density plots show two regions of interest when considering the activity of the autonomic system. LF-HRV changes during respiration and is thought to reflect sympathetic activity, whilst HF-HRV is thought to represent parasympathetic activity.25
Tilt-table testing in patients with VVS has shown a reduced BRS at rest and an exaggerated drop in BRS during HUTT.26 Changes in HRV have been less consistent in patients with VVS with the exception of LF-HRV response, which is diminished.27
The aim of orthostatic training is to modify these autonomic responses in patients to induce a normalised response to orthostatic stress and therefore preventing the symptoms of VVS.
The expected benefit in our actively-trained elderly group was not observed. One reason that might account for this is that VVS has been shown to be bi-modally distributed across the population, with peaks between20–29 years and an older cohort aged >70 years.17 This suggests that the VVS mechanism in these two groups may have some important differences that may explain the lack of efficacy of HOT seen in this trial. It is well-documented that autonomic responses are blunted in an elderly population.28 This depression of the autonomic response may account for the observed HUTT differences, with younger patients having a more pronounced and rapid-onset bradycardia with hypotension (classical response), whilst the elderly population tends to have a slower, asymptomatic onset of hypotension with a less pronounced bradycardia (dysautonomic response),29 as observed in our study cohorts. Younger patients also exhibit the classical warning signs of an exaggerated autonomic response of pallor, sweatiness, abdominal discomfort and light-headedness whilst the older population do not.4 It is therefore conceivable that orthostatic training in an elderly population may not enhance an age-related degenerated autonomic system whereas in a younger group, HOT may be able to ‘retrain’ an exaggerated or inappropriate autonomic response.
However our results show little effect of HOT in the younger control patients. The explanation for this lack of response is not clear. Previous studies have shown that the mechanism of VVS may begin to change from a predominately exaggerated autonomic response to a depressed autonomic response in patients in their forties.19 Almost two thirds of the younger cohort were aged >50 years, which may have resulted in a HOT response similar to that in the O65 actively-trained cohort. All previous studies have shown benefit from HOT in younger patients (mean ages 16.0 years,17 45.0 years,19 31.0 years22 and 19.4 years.21)
Most participants had no symptoms for the duration of the study. It has been shown that the autonomic improvements provoked by orthostatic training can be observed within 1 week of training initiation19 and that HUTT maybe rendered negative within 24 hours of in-hospital training.22 It is possible that these autonomic benefits may be lost at a similar rate on cessation of training. With a low symptom burden the participants training regime may not have been adhered to as strictly as reported.
Back pain was the most commonly reported cause of lack of HOT compliance. This was worse in the elderly cohort. In the younger group, lack of time and pre-syncopal symptoms were the most common causes of failed compliance. Overall compliance was comparable with previous studies demonstrating autonomic improvements from HOT.19
Study limitations
Participant record of compliance could not be verified beyond self-reporting, and records may be an overestimation of actual time trained. Despite this, even participants who reported great symptomatic benefit from the training showed no change in objectively assessed autonomic measures.
Conclusion
This randomised placebo-controlled study is the first to assess HOT in patients aged >65 years with VVS using serial autonomic cardiovascular reflex measures. There were no improvements in these autonomic measures in any of our study groups. The lack of efficacy in the elderly population may relate to the degeneration of the autonomic nervous system not responding to physical countermeasures. These findings do not support the use of HOT in the elderly population.