Stent thrombosis has been recognized as a serious complication of coronary stent placement since the procedure was first reported in the 1980s.1 Subsequent aggressive peri-procedural anti-thrombotic strategies with inpatient transition to warfarin reduced the risk to about 3.5 % for subacute (30-day) stent thrombosis,2,3 a rate that would be unacceptable by current standards and did not account for the yetunrecognized late and very late stent thrombosis events. The benefit of dual anti-platelet therapy (DAPT) with aspirin plus ticlopidine in association with optimal stent deployment techniques was shown to clearly reduce the risk for subacute stent thrombosis associated with bare metal stents (BMS) compared with aspirin alone or aspirin plus warfarin.4 Pooled analysis of BMS clinical trials incorporating 30 days of DAPT confirmed a subacute stent thrombosis rate <1 %, but noted a 20 % 6-month mortality for patients sustaining a stent thrombosis event.5 Eventually, clopidogrel replaced ticlopidine as a safer alternative for P2Y12 inhibition, but 30 days of DAPT remained the standard throughout the BMS era. The benefit of extending stent thrombosis beyond 30 days was first recognized following the placement of new coronary stents at the time of intracoronary brachytherapy,6 and resulted in the modification of DAPT duration from 30 days to 3 or 6 months, based on expert opinion from clinical trial Data and Safety Monitoring Committees.
Late complications were anticipated in the design of first-generation drug-eluting stent (DES) clinical trials, which also incorporated longer DAPT durations. Interestingly, although stent thrombosis between 30 days and 1 year (late) and after 1 year (very late) did occur, there was no signal of increased risk with DES compared with BMS for up to 5 years in these clinical trials.7,8 Subsequent extension of these devices to broader patient and lesion subsets in routine practice highlighted the significant problem of very late stent thrombosis,9,10 however, leading to a recommendation for further prolongation of DAPT to a minimum of 12 months.11 This recommendation was made with little evidence that 12 months was the optimal duration of DAPT. The lack of evidence along with the improved outcomes of newer-generation DES, ongoing concern about the dire consequences of stent thrombosis at any frequency, and increased recognition of the harmful impact of bleeding has resulted in the persistent question of how long (or short) a period of time DAPT should be continued for.
Reduced Risk of Late and Very Late Stent Thrombosis with Second-generation DES
In the United States, there are two classes of second-generation DES that have been approved for use: the everolimus-eluting stent (EES), marketed as Promus™ by Boston Scientific and Xience® by Abbott Vascular; and the zotarolimus-eluting stent marketed as Resolute™ by Medtronic. Several studies have demonstrated the benefits of secondgeneration DES compared with first-generation DES, with benefits sustained or increasing from 1 to 5 years.12–15 In the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) III trial of the EES versus the first-generation paclitaxel-eluting stent, at 5 years there was a significant reduction in the composite endpoint of major adverse cardiac events (13.2 % versus 20.7 %, p=0.007), including non-significant reductions in very late stent thrombosis (0.5 % versus 1.0 %, p=0.38).15 The Resolute™ all-comers trial randomized 2,292 patients to either EES or Resolute™. The participants were representative of routine practice patients. At 5 years, the overall rates of definite stent thrombosis were similar (0.8 % versus 1.6 %, p=0.084), with nearly identical low rates of very late stent thrombosis (0.5 % versus 0.4 %, p=1.00). The annual hazard beyond 1 year of 0.1–0.2 % for very late stent thrombosis was markedly lower than the 0.7 % annual hazard previously noted for first-generation DES in a similar population.10
Perhaps even more remarkable are observational studies and a network meta-analysis that have indicated rates of stent thrombosis for second-generation DES that are even lower than BMS.16,17 Although these observational data must be interpreted in the context that the BMS included were older, thicker strut devices, the comparisons were not direct, and DAPT durations were shorter for BMS, they have provided further reassurance regarding the improved safety of secondgeneration DES.
Bleeding and Impact on Mortality after Coronary Stenting
There is little doubt that bleeding has been underappreciated as a serious complication after coronary stenting and associated anti-thrombotic therapy. Earlier trials focused on rates of vascular complications and access site-related bleeding, but ongoing risks of non-access site bleeding were not reported. Part of the difficulty has been the absence of standardized criteria to define bleeding, with available definitions limited to those developed from early fibrinolytic clinical trials. The Bleeding Academic Research Consortium (BARC) has provided imperfect but standardized criteria for defining bleeding in the peri-procedural period and during follow-up.18 BARC classifies five categories of bleeding, including:18
- 1.Bleeding not requiring medical attention.
- 2.Bleeding requiring medical intervention but not meeting the criteria for categories 3, 4, or 5.
- 3.Bleeding associated with a hemoglobin drop >3 g or requiring transfusion or related to cardiac tamponade, intracranial hemorrhage, or intraocular hemorrhage.
- 4. Bleeding related to cardiac surgery.
- 5. Fatal bleeding.
Rates of bleeding after coronary stenting and during DAPT vary according to the baseline risk of the population, with older age, female gender, history of hypertension, renal insufficiency, concomitant oral anticoagulants, and acute coronary syndrome being indications predictive of higher bleeding risk in most studies.19–21 Bleeding related to the access site as well as non-access site bleeding has been associated with subsequent mortality, although the mortality risk appears to be highest for later, spontaneous bleeding.21–23 Multiple studies have now demonstrated that the risk of mortality associated with bleeding after coronary stenting is at least as great as that related to myocardial infarction (MI).21,24,25 Furthermore, the risk of bleeding associated with coronary stenting and DAPT is ongoing throughout the duration of DAPT, even among patients who remain free of bleeding events for the first 12 months.26,27
Evidence Supporting Shorter DAPT
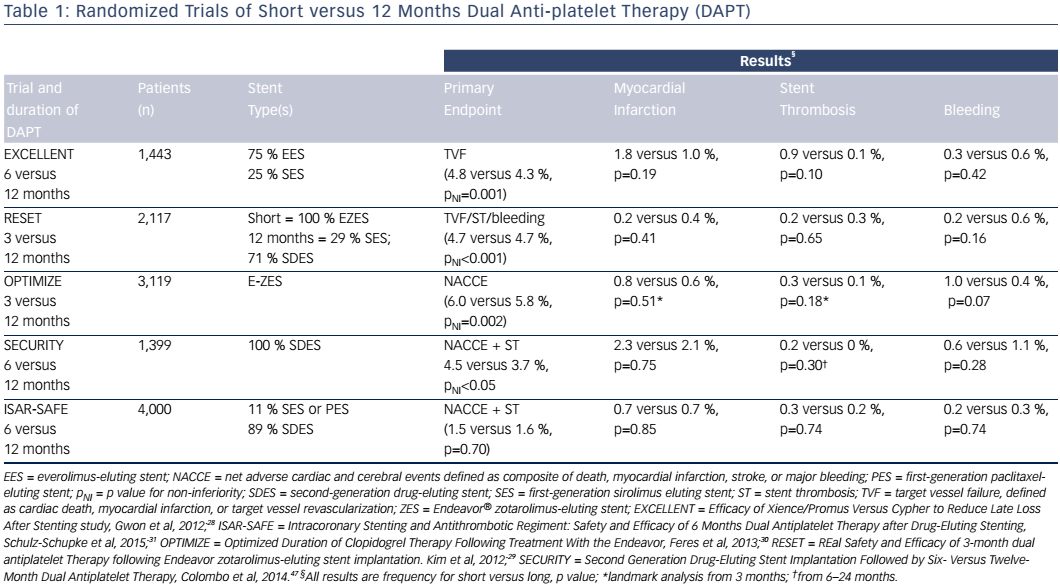
The proven safety advantage of second-generation DES and the increasing awareness of the consequences of bleeding related to prolonged DAPT have fueled interest in shorter durations of DAPT. Randomized clinical trials that have compared DAPT durations as short as 3 months with 12 months of DAPT are shown in Table 1.28–31 Generally, these trials were designed to show non-inferiority in composite endpoints that included ischemic or bleeding events, and in some cases repeat revascularization events. Except for the Intracoronary Stenting and Antithrombotic Regiment: Safety and Efficacy of 6 Months Dual Antiplatelet Therapy after Drug-Eluting Stenting (ISAR-SAFE) study, the trials initiated randomization at the time of the index procedure, possibly limiting enrollment of higher-risk patients.31 ISAR-SAFE patients who remained free of events at 6 months were eligible for randomization. None of the studies comparing durations of DAPT were powered to assess differences in MI or stent thrombosis, and event rates were low in both the short and 12-month DAPT groups, consistent with the enrollment of lower-risk patients. ISARSAFE, which was powered to assess meaningful differences in ischemic endpoints, was terminated early due to low event rates and slow enrollment,31 suggesting that there may have been selection of low-risk patients for randomization in this trial as well.
Other indirect evidence for the success of shorter DAPT duration for newer-generation DES comes from the Prospective Randomized Comparison of the BioFreedom Biolimus A9 Drug-Coated Stent versus the Gazelle Bare-Metal Stent in Patients at High Bleeding Risk (LEADERSFREE) trial.32 The BioFreedom™ device does not include a polymer for drug delivery and becomes a bare metal stent within 30 days. Patients at high risk of bleeding were randomized to the polymer-free DES or the identical BMS, and all patients were prescribed 30 days of DAPT. At 1 year, the DES cohort had a lower rate of cardiac death, MI or stent thrombosis (9.4 % versus 12.9 %, p=0.005), the primary endpoint, owing to reduced MI (6.1 % versus 8.9 %, p=0.01).32 The stent used in this study has much thicker struts (120 μm) than contemporary BMS or DES, which is a concern as it may have contributed to an increased risk of restenosis and stent thrombosis. This may also explain the high MI rate in the BMS group – nearly one-third of cases were directly related to restenosis – and also account for the much higher than expected rate of stent thrombosis in both groups (>2 % at 1 year).32
Taken together, these data support the safety of shorter DAPT duration in some patients, but are not conclusive for adopting DAPT <12 months as a routine strategy for all patients.
Evidence Supporting Longer DAPT
Prior to current data showing low (0.1–0.2 %/year) rates of very late stent thrombosis with second-generation DES, most of the debate on DAPT duration was about whether it should be >12 months. Given the serious consequences of stent thrombosis and the increasing complexity of patients and lesions undergoing coronary stenting, with likely higher risk for very late stent thrombosis, the relative safety of longer-term therapy has remained a topic of interest. The question was addressed by three earlier randomized trials.33–35 Unfortunately, none of these trials was adequately powered to address meaningful differences in ischemic endpoints, in particular very late stent thrombosis. For example, in the Prolonging Dual Antiplatelet Treatment After Grading Stent-induced Intimal Hyperplasia (PRODIGY) study, very late definite or probable stent thrombosis occurred in 6/983 (0.6 %) patients assigned to 6 months of DAPT and 3/987 (0.3 %) patients assigned to 24 months of DAPT.35 With these observed rates and sample size, the study failed to exclude as much as an eightfold higher risk for very late stent thrombosis. The three randomized trials did, however,confirm consistently higher bleeding rates with longer-term DAPT.33–35 In PRODIGY, for example, major bleeding, defined as BARC 3 or 5, occurred in 3.4 % versus 1.9 % of patients (p=0.037).35
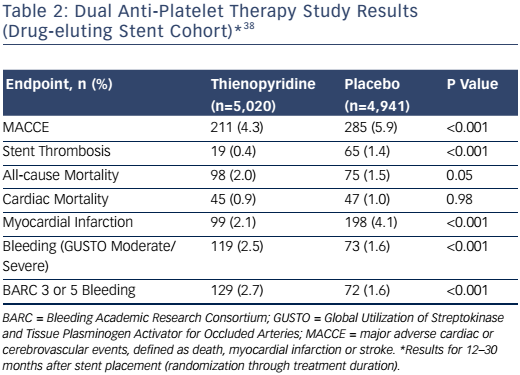
The safety and effectiveness of extending DAPT beyond 12 months was addressed more definitively in the Dual Anti-Platelet Therapy study.27 This study was conceived at the height of the concern over very late stent thrombosis with first-generation DES, but the rapid changes in practice and duration of enrollment required for this large, international trial resulted in nearly half of the population receiving second-generation DES. The study enrolled over 25,000 patients, including 22,866 who received a DES. After 12 months of prescribed DAPT plus aspirin, 9,961 of these patients who were compliant with DAPT and free from ischemic or bleeding events were randomized to aspirin plus placebo or to DAPT for an 18 additional months. Another group of 1,687 patients who had received only BMS were also randomized to continued thienopyridine or placebo. Unlike the previous long-term trials, the Dual Anti-Platelet Therapy study was powered to address meaningful differences in a composite ischemic endpoint of major adverse cardiac and cerebrovascular events, defined as death, stroke or MI, as well as a co-primary endpoint of definite or probable stent thrombosis. Bleeding classified as moderate or severe by Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries (GUSTO) criteria was the major safety endpoint. The primary results for the DES cohort are shown in Table 2.
The co-primary endpoints of major adverse cardiac and cerebrovascular events and stent thrombosis were each significantly lower for patients who continued DAPT for between 12 and 30 months.27 Notably, the absolute difference in risk for MI was greater than that for stent thrombosis, as approximately half of the reduction was for MI unrelated to a stent thrombosis event. The overall benefit of continued DAPT for reducing stent thrombosis was consistent across a number of subgroups, including patients receiving only BMS (0.5 % versus 1.1 %; hazard ratio 0.49; 95 % CI [0.15–1.64]; p=0.42) and those who received a second-generation EES (0.3 % versus 0.7 %; hazard ratio 0.38; 95 % CI [0.15–0.97]; p=0.76).27 The absolute differences in risk, however, were smaller in these subgroups, indicating a greater number needed to treat to avoid a single event.
As in other studies, the Dual Anti-Platelet Therapy study demonstrated that benefits in ischemic endpoints must be balanced against the increased risk of bleeding. This risk was noted whether defined by GUSTO or BARC criteria, and despite the exclusion of patients on oral anticoagulants and those who had a bleeding event during the 12 months prior to randomization.27 Another concern from the Dual Anti-Platelet Therapy study was the unexpected increase in overall mortality related to an increase in non-cardiac mortality without being offset by reduced cardiac mortality. Although detailed post-hoc analyses did not confirm a significant association between bleeding and non-cardiac mortality, there was a numerical increase in deaths due to either bleeding or trauma and cancer-related deaths. A later meta-analysis of clinical trials comparing shorter versus longer (>12 months) duration of DAPT among DES patients also reported higher non-cardiac mortality.36
The Dual Anti-Platelet Therapy study specified 30 months’ DAPT duration based on protocol design, but it is notable that in the 3 months after discontinuation of thienopyridine, there was an increase in the risk of MI and stent thrombosis, similar to that observed in the 12–15-month interval for the placebo group.27 It is uncertain whether this represents a need for even longer DAPT duration in some patients, or whether it is a possible effect of thienopyridine withdrawal.
Balancing the Risks of Bleeding and Ischemia
The optimal duration of DAPT must balance the risks of bleeding and ischemic events, each of which carry a substantial hazard for subsequent mortality. Although stent thrombosis carries the highest mortality risk in the subsequent 2 years after the event, especially if occurring within the first 30 days, it occurs much less frequently than overall MI or bleeding, such that the attributable risk is similar for combined ischemic and bleeding events.37 Several reports have indicated that baseline characteristics may help discriminate these risks for individual patients and allow for a personalized approach based on clinical and procedural risk rather than indiscriminate short or long durations.38–41
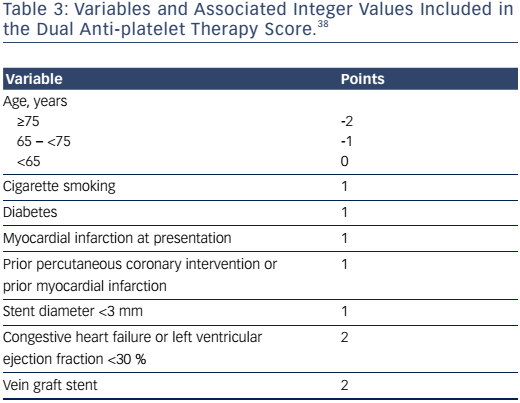
In the Dual Anti-Platelet Therapy study, a score was developed based on models that predicted risk of ischemia (MI or stent thrombosis) or GUSTO or moderate or severe bleeding (see Table 3).38 A sum of the factors generated a composite score with range from -2 to 10 that indicated net benefit or harm with continued thienopyridine. For example, for DAPT scores below the median (2), the continued use of thienopyridine compared with placebo after 12 months was associated with increased bleeding (3.0 % versus 1.4 %, p<0.001) and an insignificant reduction in ischemia (1.7 % versus 2.3 %, p=0.07); whereas the difference in risk among patients with a high score (≥2) was highly significant for ischemia (2.7 % versus 5.7 %, p<0.001) but not for bleeding (1.8 % versus 1.4 %, p=0.26). This score is limited by the design of the Dual Anti-Platelet Therapy study, which excluded patients at the highest risk for bleeding and those with bleeding or ischemia in the first 12 months after stenting.
The Patterns of Non-adherence to Anti-platelet Regimens in Stented Patients (PARIS) registry investigators also tabulated risk scores for ischemia or major bleeding based on baseline clinical characteristics.39 Older age, anemia, and requirement for oral anticoagulants were unique predictors for bleeding; while diabetes, acute coronary syndrome indication, and previous revascularization were unique predictors for ischemia. Renal insufficiency and current smoking increased the risk of both bleeding and ischemia.
Of note, acute coronary syndrome presentation is a significant predictor of recurrent ischemia in both the DAPT and PARIS scores. Furthermore, in a recent meta-analysis, longer duration of DAPT (>6 months) was associated with reduced risk of MI or stent thrombosis among acute coronary syndrome patients but not stable coronary artery disease patients.40
Lesion and procedural complexity may also be important determinants of risk versus benefit for longer DAPT duration. In a patient-level meta-analysis of clinical trials comparing DAPT for 3 or 6 months with 12 months or longer, each component of complex percutaneous coronary intervention (three-vessel treatment, ≥3 stents implanted, ≥3 lesions treated, bifurcation treated with two stents, total stent length >60 mm, or chronic total occlusion) was associated with reduced major adverse cardiac events (defined as death, MI or stent thrombosis) for longer-term DAPT.41 The magnitude of the difference in risk increased with the number of complex percutaneous coronary intervention components present.
Patients requiring continued oral anticoagulants are at especially high risk for bleeding with prolonged DAPT. The optimal duration of DAPT and whether it should be abandoned in favor of single anti-platelet therapy with a P2Y12 inhibitor but no aspirin for a period of 1–12 months remain topics of debate and clinical study. There is evidence from the What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing (WOEST) trial that a regimen of continued oral anticoagulants with clopidogrel alone versus clopidogrel plus aspirin reduced major bleeding (GUSTO moderate or severe, 5.4 % versus 12.3 %, p=0.003) and all-cause mortality (2.5 % versus 6.3 %, p=0.027), without an increase in MI or stent thrombosis, but the study was not powered to assess for differences in ischemic outcomes.42 More recently, the Study Exploring Two Strategies of Rivaroxaban and One or Oral Vitamin K Antagonist in Patients with Atrial Fibrillation who undergo Percutaneous Coronary Intervention (PIONEER AF-PCI) results were published. Investigators reported that among patients with atrial fibrillation undergoing coronary stenting a reduced dosage of rivaroxaban (15 mg daily) plus a P2Y12 inhibitor (95 % clopidogrel) for 12 months or very low dose rivaroxaban (2.5 mg twice daily) plus DAPT for 1, 6 or 12 months followed by rivaroxaban 15 mg daily plus aspirin 75–100 mg daily for total of 12 months of therapy had reduced bleeding compared with warfarin plus DAPT for 1, 6, or 12 months followed by warfarin plus aspirin 75–100 mg daily.43 The three groups had similar rates of cardiovascular death, MI and stroke, but the study was not powered to exclude differences in these outcomes. These and other potential regimens including very short DAPT (1 month) or other novel oral anticoagulants with single anti-platelet agents provide numerous options for reducing bleeding events, but remain unproven for reducing stent thrombosis or MI. It is likely that even among patients who require continual oral anticoagulants, other clinical or procedural factors that increase the risk for stent thrombosis or MI may argue for longer or more aggressive anti-platelet strategies as part of a personalized strategy in some patients.
Current Society Guidelines and Options for Personalized Strategy
The most recent guidelines from the European Society of Cardiology (ESC) and the American College of Cardiology and American Heart Association (ACC/AHA) incorporate options for shorter DAPT duration for patients deemed to be at higher bleeding risk, and longer duration (>12 months) for patients with low bleeding risk and high risk for ischemia.44–46 For patients with coronary stenting for stable CAD, the guidelines advise a minimum DAPT duration of 1 month for BMS and 6 months for DES if patients are not at a high risk of bleeding. If they have a high bleeding risk, the ACC/AHA guidelines allow for a shorter duration of 3 months after DES, while the ESC guidelines include options of 1 month of DAPT or initial dual therapy with clopidogrel plus an oral anticoagulant in DES patients with the highest bleeding risk and a requirement for oral anticoagulants. Both sets of guidelines also consider it reasonable to extend therapy beyond 6 months if patients have a high risk of ischemia and low risk of bleeding.
After stenting for acute coronary syndromes, the ACC/AHA guidelines advise 12 months of DAPT, with a consideration to shorten this to 6 months if a patient is at high bleeding risk or extend this beyond 12 months if a patient has tolerated DAPT without bleeding and remains at low risk for bleeding.45–46 The ESC guidelines are similar, except they advocate the consideration of shortening of DAPT duration to 3 months if a patient is at high bleeding risk.44
Conclusion
DAPT after coronary stenting along with optimal stent deployment techniques has led to low rates of stent thrombosis. Concern about the ongoing risk of stent thrombosis beyond 1 year has been clearly reduced with the introduction of second-generation devices. Further reduction in both stent thrombosis and overall MI can be achieved with extended DAPT duration beyond 1 year. The unavoidable trade-off between risk of bleeding and possibly increased overall mortality with longer DAPT duration requires a careful consideration of the net benefit of continuing therapy beyond the minimum time necessary. A personalized strategy based on the balance of these risks rather than an indiscriminate approach adopting short- or long-term DAPT is required for optimal outcomes. Future studies should determine the necessary minimum durations for current and new devices and help to clarify algorithms for determining the absolute risk of ischemia versus bleeding, and thus the net clinical benefit of longer-term therapy.