Intracoronary imaging is able to aid the interventional cardiologist in the characterisation of atherosclerotic plaque morphology, in optimising stent sizing, and in minimising the complications associated with percutaneous coronary intervention (PCI). Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are commonly used methods, while newer spectroscopic methods are under development.
Intravascular Ultrasound Versus Optical Coherence Tomography: Technology
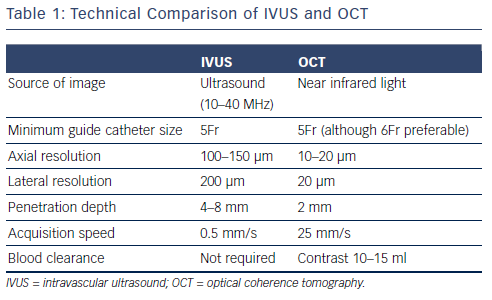
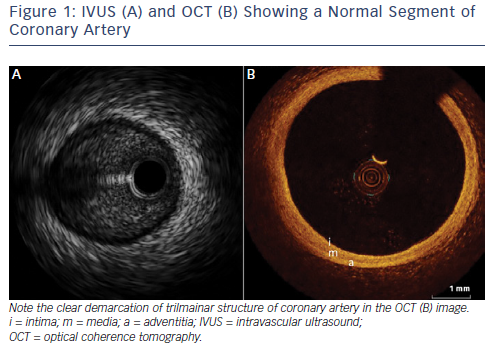
Table 1 displays a technical comparison of the IVUS and OCT imaging methods. The principle of IVUS imaging is based on the ultrasound waves produced by the oscillatory movement of a transducer.1 Commercially available IVUS systems have transducers mounted on catheters that are compatible with guiding catheters in sizes of 5Fr or larger. These catheters can be inserted into the coronary artery over a 0.014 inch conventional guide wire and imaging can be obtained by manual or motorised pullback. Motorised pullbacks are carried out at a speed of 0.5 mm/s, thus a 50 mm coronary artery can be imaged in approximately 90s. Integrated IVUS consoles add to the rapidity of imaging, but mobile IVUS carts are also available. When co-registration of IVUS with angiography becomes available, this will be a useful adjunct in locating the anatomical lesion precisely. Once the pullback is recorded, measurements of the lumen can be carried out either manually or using automated software. Greyscale IVUS has an axial resolution of 100–150 μm, lateral resolution of 200 μm1 and penetration depth of 4–8 mm. Post-processing of greyscale IVUS images is possible with radiofrequency-based technology such as IVUS virtual histology.2 IVUS virtual histology may assist in characterisation of plaque morphology by differentiating between various types of plaque using colour coding. As a result of limited resolution, IVUS cannot reliably identify the separation between intima and media and the relation between adventitia and peri-adventitial structures (see Figure 1).3
In contrast to IVUS, intracoronary imaging by OCT is obtained using near-infrared light. The first generation of OCT imaging was based on occlusive balloon technology called time domain (TD) imaging. Use of frequency domain (FD) imaging, also referred to as Fourier domain spectral imaging, has now surpassed TD imaging.4 FD imaging does not require occlusion of the proximal artery with a balloon as high viscosity liquids such as contrast media can be used to purge blood from the vessel, while imaging is completed rapidly. Current commercially available OCT catheters consist of a single-mode optical fibre in a hollow metal wire torque that rotates at a speed of 100 rps. The axial and lateral resolutions of OCT are 10–20 μm and 20 μm, respectively – which is superior to that of IVUS. However, better resolution comes at a drawback of limited penetration – a maximum of 2 mm.5 With acquisition speeds of up to 25 mm/s, rapid imaging of coronary artery can be achieved within a few seconds. Commercially available OCT catheters can be inserted into coronary artery on a 0.014 inch guide wire and are compatible with guiding catheters sized 5Fr or larger. For optimal imaging quality, a bloodless field is required, which can be achieved with injection of 12–15 ml of highly viscous medium such as contrast, either by hand or by use of a power injector. Blood clearance can be challenging through a 5Fr catheter, therefore 6Fr or larger is generally recommended. Caution needs to be exercised in people with renal impairment where multiple pullbacks are contemplated due to the risk of contrast nephropathy.
Current commercially available OCT software automatically detects the lumen, allows marking of every frame and gives user-defined proximal and distal reference frames with dimensions. Furthermore, every pullback of the coronary artery can be viewed in cross-sectional frames (see Figure 1), longitudinal view and lumen profile view. Cross-sectional views are helpful in detailed study of plaque where as longitudinal and lumen profile views can be used for longitudinal measurements such as stent length. 3D reconstruction is possible and may assist in detailed assessment of bifurcation lesions and in optimising PCI results. Co-registration of OCT with angiography can be a useful adjunct in locating anatomical lesions precisely, reducing the chances of geographical miss.
Intravascular Ultrasound Versus Optical Coherence Tomography: Safety and Feasibility
One of the limitations of earlier TD-OCT imaging was the requirement to have a bloodless field for adequate imaging. This was achieved by proximal occlusion of the coronary artery with a semi-compliant balloon followed by flushing of the artery with ringer’s lactate solution. Although this method was not associated with any serious complications, minor complications such as transient ST elevation with associated chest pain were common.6 Furthermore, the acquisition time was longer with a pullback speed of only 0.5–3.0 mm/s. In comparison, current FD-OCT imaging does not require balloon occlusion of coronary artery and, with higher pullback speeds, a bloodless field can be achieved by contrast injection.
Both IVUS and current-generation FD-OCT have been shown to have a favourable safety profile. An integrated biomarker and imaging substudy (IBIS-4) assessed the feasibility and the procedural and longterm safety of OCT and IVUS in patients with ST-elevation MI (STEMI) undergoing primary PCI.7 In this prospective cohort study, 103 patients with STEMI who underwent serial three-vessel coronary imaging during primary PCI were compared with 485 patients with STEMI undergoing primary PCI without additional imaging after 13 months. Feasibility (defined as the number of pullbacks suitable for analysis) and safety (defined as the frequency of peri-procedural complications and major adverse cardiac events [MACE, a composite of cardiac death, MI and any clinically indicated revascularisation at 2 years]) outcomes were recorded. Successful imaging was achieved in <90 % of patients at baseline and follow-up using IVUS and OCT. Although peri-procedural complications occurred with OCT imaging (<2.0 % versus 0 % with IVUS), long-term safety was favourable with both modalities, with no significant difference in MACE rates at 2 years.7 Of note, the majority of OCT-related complications were transient ST elevation due to coronary spasm, which in clinical practice can be mitigated by administering intracoronary nitrates prior to imaging.
In another registry-based study, OCT and IVUS were shown to have comparable safety and feasibility profiles.8 Analysis of 3,045 OCT pullbacks from 1,142 patients and 5,148 IVUS pullbacks from 2,476 patients revealed seven complications related to OCT and 12 to IVUS imaging. Transient ST-elevation requiring withdrawal of the imaging catheter was noted with OCT, whereas IVUS appears to have been associated with coronary spasm, thrombus formation, dissection of the imaged vessel and stent deformation.8
Is One Choice of Intracoronary Imaging Superior to the Other and What are the Clinical Scenarios Where They Should be Used?
Intravascular Ultrasound
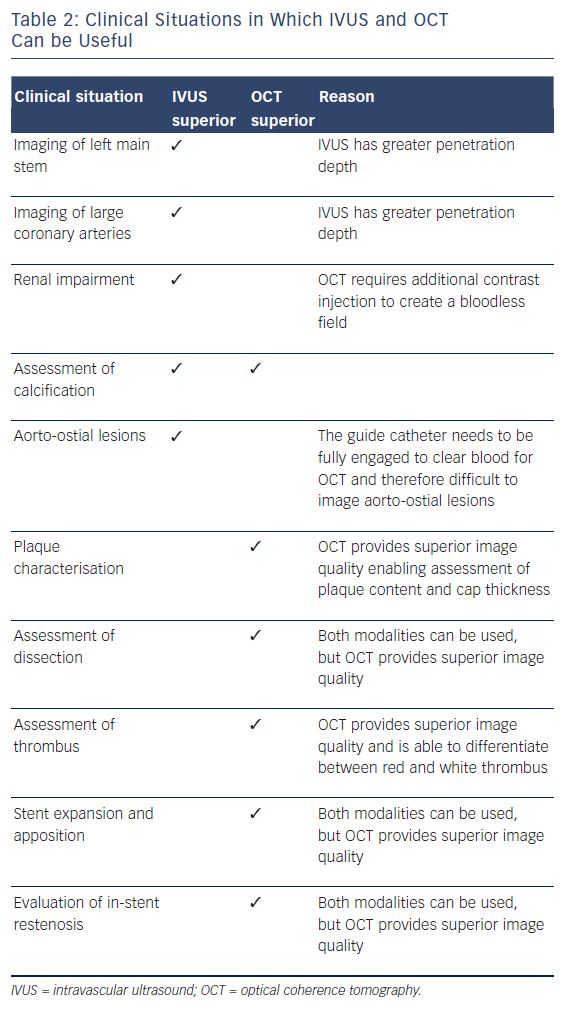
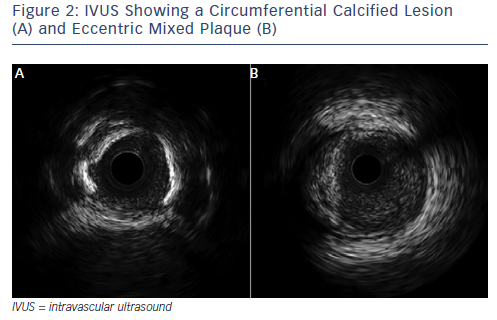
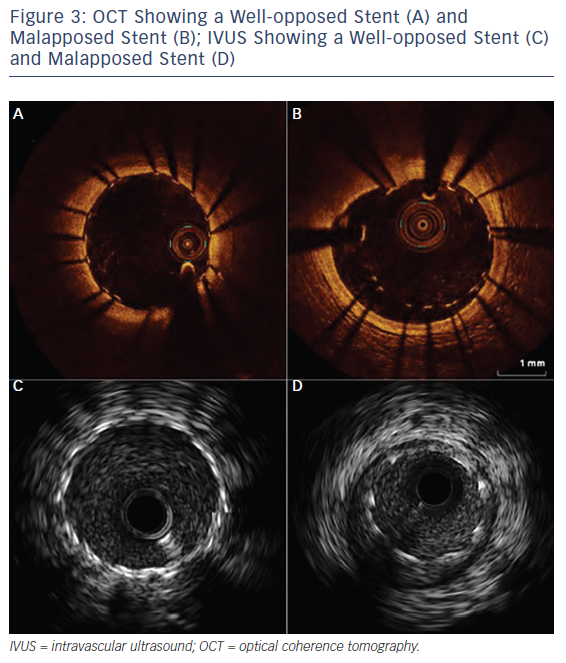
Over the past two decades, IVUS has become the reference tool for intracoronary imaging. Advances in IVUS technology have resulted in better resolution and penetration: IVUS can now be used to assess plaque characteristics, volume and constituents (see Figure 2; Table 2).9 Coronary arterial and lumen dimensions, particularly minimal luminal area (MLA), can be measured accurately by IVUS algorithms, which can assist in the decision-making process for revascularisation.10 One of the most important roles of IVUS is in optimising PCI, particularly in complex lesions subsets such as left main stem (LMS), calcific and bifurcation lesions.11 IVUS can be used to optimise PCI as it has a role in stent sizing and in detecting adequate stent expansion and strut malapposition (see Figure 3).12 IVUS, with its better penetration, is superior to OCT in assessing the remodelling patterns of the vessel wall. IVUS-detected positive vessel remodelling of the coronary artery is associated with late stent thrombosis following drug-eluting stent (DES) implantation.13
Optical Coherence Tomography
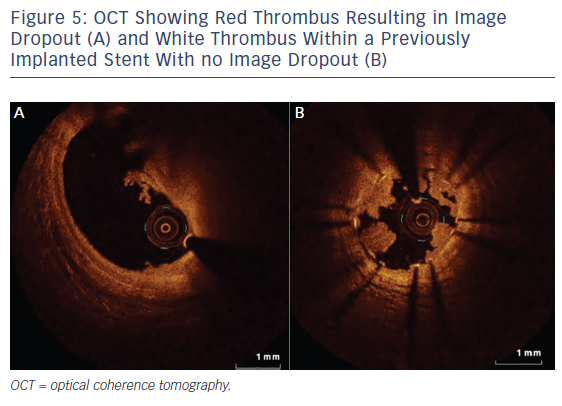
OCT, with even better resolution when compared with IVUS, can be used in assessing plaque characteristics14 and constituents15 and in optimising PCI.16 However, with limited penetration, assessment of plaques with thickness of >1.0–1.5 mm is not possible with OCT.4,17 Up to 25 % of acute coronary syndrome events are secondary to thrombus present on a non-ruptured plaque, also called an erosion.15,18 OCT helps in accurately identifying eroded plaques, where if no lumen narrowing is present stenting may not be needed. The presence of thin-cap fibroatheroma (TCFA), defined as lipid plaque thickness of <65 μm, is predictive of future adverse cardiac events.18 However, interpretation of TCFA on OCT requires caution as artefacts due to tangential dropout can lead to misinterpretation.19 The superior resolution of OCT means it can detect TCFA with adequate sensitivity/ specificity,20 and also detects the presence of macrophages21 and neovessels, and lipid volume – the features of so-called vulnerable plaque (see Figure 4). Furthermore, OCT not only identifies the presence of thrombus, but can also distinguish between red and white thrombus often seen in STEMI (see Figure 5; Table 2).20 Injury to the vessel wall post-PCI reflected by the presence of intimal tears, edge dissections, tissue prolapse, presence of thrombus and incomplete stent apposition can be readily assessed by OCT, which allows for optimisation, as required.16 Two further areas where imaging with OCT is useful are capability of detecting thin neo-intima in follow-up imaging after DES implantation22 and in delineating tissue characteristics of in-stent restenosis (see Figure 4).23 OCT is superior to IVUS in identifying uncovered stent struts. Sub-analysis of the Optical Coherence Tomography for Drug Eluting Stent Safety (ODESSA) trial showed 8 % of the stented segments with no detectable neointima by IVUS were found to have neo-intimal coverage by OCT.24
Is There Evidence Supportive of One Modality Over the Other?
In the field of interventional cardiology, any new diagnostic tool or treatment modality needs to be associated with better clinical outcomes before being incorporated into guidelines and adapted widely in the clinical environment. Here, we review the data supporting the use of these imaging techniques in contemporary clinical practice.
Intravascular Ultrasound
A meta-analysis of IVUS-guided PCI by Zhang et al. showed improved clinical outcomes.25 In their analysis, over 19,000 patients across eleven studies (one randomized controlled trial and 10 registries) were included. Compared with angiography alone, IVUS-guided DES implantation was associated with reduced rates of death, MACE and stent thrombosis. No difference was found in the rates of MI, target lesion and target vessel revascularisation.

The non-randomised Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents (ADAPT-DES) study analysed 3,349 patients in whom IVUS was used to guide PCI.26 IVUS guidance changed the PCI strategy in 74 % of cases. IVUS guidance compared with angiography alone was associated with reduced 1-year rates of stent thrombosis, MI and MACE following DES implantation.
IVUS can be a useful adjunct in the assessment of LMS disease. IVUS generated MLA correlates with fractional flow reserve (FFR) in the absence of proximal left anterior descending and circumflex artery disease. IVUS MLA of <5.9 mm2 correlates well with FFR of <0.75.27 However, caution need to be exercised in Asian patients who have smaller coronary arteries. Kang et al. showed that an MLA cut-off of <4.1 mm2 correlated with FFR <0.7528 in a Korean population. In non- LMS lesions, the correlation of IVUS derived MLA with FFR is weak with limited accuracy.29 The reasons for this are lesion location in the coronary tree, lesion length, eccentricity, entrance and exit angles, shear forces, reference vessel dimensions, and the amount of viable myocardium subtended by the lesion.30 Although it is safe to defer PCI in non-LMS lesions with MLA >4 mm2, lesions with MLA <4 mm2 may need to be physiologically tested before intervention.31
Optical Coherence Tomography
Currently trial data looking at OCT use and clinical outcomes are limited. The Centro per la Lotta contro l’Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study compared outcomes between patients undergoing PCI under angiography guidance alone versus angiography plus OCT guidance.32 The group that underwent PCI with angiography and OCT guidance had overall significantly lower rates of cardiac death, MI and repeat revascularisation. Furthermore, OCT revealed adverse features following PCI in almost 35 % of patients who needed further intervention.
The Observational Study of Optical Coherence Tomography in Patients Undergoing Fractional Flow Reserve and Percutaneous Coronary Intervention (ILUMIEN) I study assessed how the clinical decisionmaking process is influenced when OCT is added to angiography and FFR.33 This study enrolled 418 patients scheduled for PCI from 35 international centres, including patients with stable and unstable coronary syndromes, prospectively in a non-randomised fashion. Once recruited, the majority of patients underwent pre-PCI FFR and OCT imaging. OCT imaging influenced physician decision-making processes pre-PCI in 57 % and post-PCI in 27 % of cases. Additional in-stent post-dilatation was carried out in 81 % and additional stent placement in 12 % of the cases. Device-oriented MACE (cardiac death, MI and target lesion revascularisation) and patient-oriented MACE (all-cause death, MI and any repeat revascularisation) were rare in hospital and at 30 days. The rates of other events such as stent thrombosis were also extremely low.33 The ILUMIEN II study showed the degree of stent expansion achieved after OCT versus IVUS guidance to be comparable.34 In this retrospective study, propensitymatched analysis of 354 patients who underwent OCT in the ILUMIEN I trial and 586 patients from the IVUS substudy of the ADAPT-DES trial based on reference vessel diameter, lesion length, calcification, and reference segment availability was comparable between OCT and IVUS guidance, as were the rates of major stent malapposition, tissue protrusion, and stent-edge dissection.34 The ongoing Optical Frequency Domain Imaging Versus Intravascular Ultrasound In Percutaneous Coronary Intervention (OPINION – OFDI) and ILUMIEN III randomised trials will further help in elucidating the potential of OCT versus IVUS in optimising PCI outcomes. The OPINION – OFDI study has completed recruitment with 800 patients divided equally between OCT and IVUS arms.35 The preliminary results showed comparable safety profiles and stent expansion with OCT and IVUS guidance immediately after PCI. The follow-up results at 1 year, including outcome data, are awaited.
As with IVUS, the OCT-derived MLA of 1.9 mm2 correlates well with an FFR of <0.75.36 Another study of 56 stable patients reported OCTderived MLA of 1.95 mm2 correlating well with an FFR of <0.80, with a sensitivity of 82 % and specificity of 63 %. However, 5 of the 26 patients with MLA >1.95 mm2 had an FFR of <0.80, suggesting that OCT cannot be a surrogate for FFR.37
Guidelines
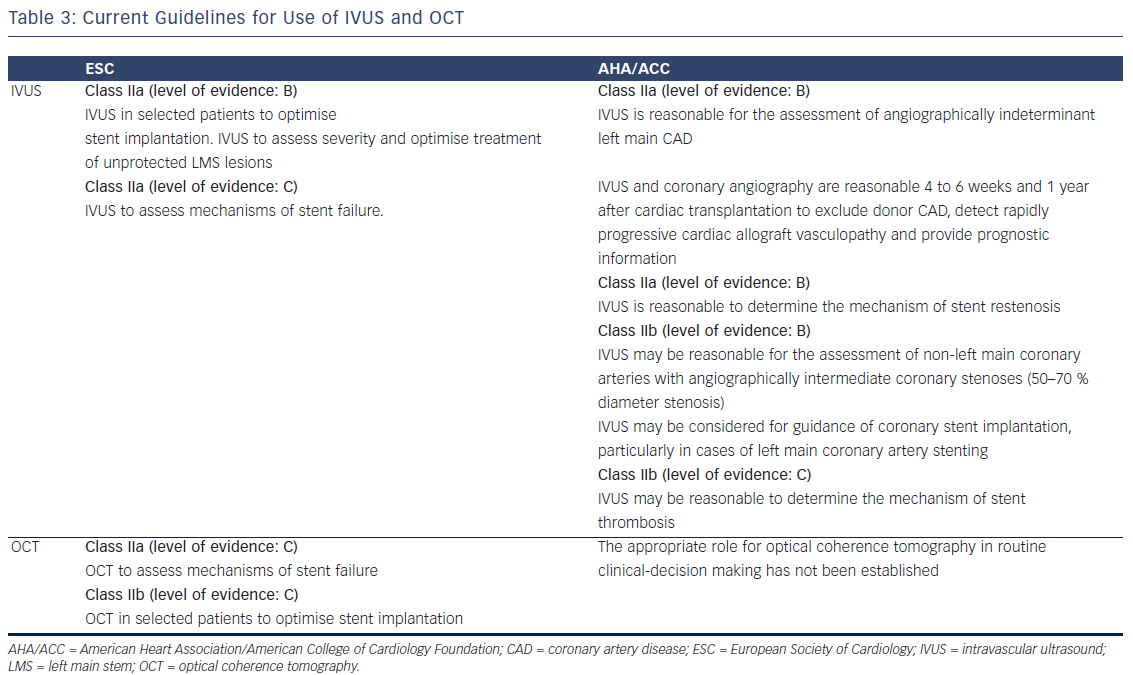
The American College of Cardiology Foundation/American Heart Association/Society for Cardiac Angiography (ACC/AHA/SCAI)31 and European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS)38 guidelines on myocardial revascularisation have issued a class II recommendation for IVUS with varying levels of evidence depending on the indication (see Table 3). OCT, Owing to a lack of clinical data, OCT is not included in the US guidelines, whereas the European guidelines have given a class II recommendation for OCT (see Table 3).
Future Directions
It is evident that both technologies have advantages and limitations. More technological adaptions are under way to enhance the use of IVUS and OCT. For example, OCT equipment that can complete pullback of entire coronary artery with in one heartbeat to minimise artefacts is undergoing experiments.39 Micro-OCT that has the ability to study endothelium and macrophages in vivo in detail is also under development.40 Experiments looking into feasibility of photo acoustic imaging in humans one are also underway.41
Conclusion
Intracoronary imaging has given a new dimension to the field of interventional cardiology. When choosing the modality of intracoronary imaging, the anatomic location in the coronary tree appears to be a good discriminator. IVUS has better data when it comes to LMS-related lesions, whereas OCT seems to be superior in arteries with an MLA of <3 mm2. When it comes to establishing diagnosis and optimising stent deployment, OCT has the advantage of better resolution. However, when it comes to assessing the significance of intermediate coronary stenosis, physiological assessment with FFR should remain the first choice as IVUS- and OCT-derived MLA cut-off values have at best moderate correlation and accuracy.
IVUS and OCT are safe and feasible to use in modern cardiac catheter laboratory practice. In the hands of an experienced operator, imaging can be done rapidly with minimal or no complications. With advantages and limitations of one modality over the other, intracoronary imaging with IVUS and or OCT have the potential to complement each other. Future data justifying their routine use based on improved clinical endpoint data is forthcoming. The future of intracoronary imaging is likely to incorporate co-registration with angiography as standard, hybrid and molecular imaging. Future technological advances in intracoronary imaging provide further exciting opportunities for a better understanding of the coronary disease process and response to revascularisation.