Progressive thickening of the aortic valve leaflets and narrowing of the aortic annulus leads to increased mechanical stress on the left ventricle and reduces cardiac output, resulting in further complications.1–3 The proportion of the population affected increases as the median age of a country or region rises. Approximately 2–4 % of people aged over 65 will develop calcific aortic stenosis, with 25 % of people in this age group presenting with signs of the disease, leading to a 50 % increased risk of cardiovascular related events. Furthermore, there is an associated risk of 80 % over 5 years of progression to heart failure, aortic valve replacement or death.4
Anatomy and Histology
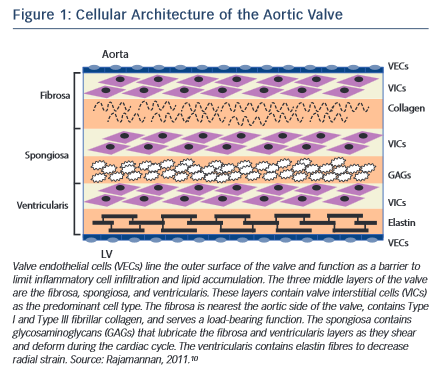
The normal aortic valve maintains unidirectional blood flow from the left ventricle into the aorta. It is a supple membrane that opens and closes with each heartbeat more than 100,000 times a day. The healthy aortic valve comprises three leaflets and is located at the junction between the left ventricular outflow tract and the aortic root. The internal collagen framework of the leaflets is arranged in three distinct layers, which – from the aortic to ventricular surface – are the fibrosa, spongiosa, and ventricularis (see Figure 1 ). This leaflet structure is covered on both the ventricular and aortic surfaces by endothelium in continuity with both the ventricular endocardium and the aortic endothelium. Each layer of the aortic valve has a distinct structure and function. The fibrosa, with its dense connective tissue, contains circumferentially oriented collagen fibres that provide most of the strength of the leaflets. The spongiosa is found at the bases of the leaflets. It contains a loose matrix of mucopolysaccharides, and provides a cushion to resist compressive forces and facilitate movements between the fibrosa and ventricularis during leaflet motion. The ventricularis layer contains radially oriented elastin and contributes to flexibility, allowing for changes in leaflet shape during opening and closing. Under normal conditions, all three layers are avascular with no cellular infiltrates and are innervated by adrenergic and cholinergic neural networks.5–7 To remain pliable, the aortic valve must undergo continuous repair throughout life. Accumulation of fibrotic tissue and calcium in a valve leads to decreased pliability and narrowing of the valve orifice.8,9
Valve interstitial cells (VICs) are found in each of these layers, and have distinct sub-populations that regulate homeostasis within the valve leaflets.10–12 In addition to the common tricuspid anatomy of the aortic valve, a congenital bicuspid valve is found in 0.5–1.4 % of the general population, giving rise to differential biomechanical forces – both on the valve and the aortic wall.13–15
Pathophysiology and Mechanism of Calcification
Over the past several decades, the aetiology of calcific aortic valve disease (CAVD) has changed considerably. The lower prevalence of rheumatic heart disease and increased longevity in industrialised countries has resulted in a pattern shift from rheumatic to degenerative calcification as the most common cause of CAVD and subsequent calcific aortic stenosis.16–18 CAVD is the third most common heart disease in the western world,19 following coronary heart disease and hypertension. Its prevalence in the elderly (≥65 years of age) ranges from 2–4 % when considering only severe aortic stenosis, increasing to 25 % when aortic sclerosis is included.9 However, a relative minority of elderly individuals develop aortic valve calcification, suggesting pathological influences other than age play a role.
Calcific aortic stenosis is the second most prevalent cause for heart surgery and is responsible for approximately 15,000 deaths annually in North America.18 Calcific aortic stenosis is a well-known disease entity and we are able to assess numerous haemodynamic parameters using cardiac catheterisation or ultrasonography as well as cardiac computed tomography and cardiac magnetic resonance imaging.20 In CAVD, calcified nodules are initially observed at the base of the cusps and their presence gradually extends towards the orifice. All three cusps are usually usually affected, but one or more may be dominant. When blood flow through the stenotic aortic orifice becomes significantly restricted, haemodynamic impairment associated with serious symptoms of congestive heart failure and sudden cardiac death may occur. Severe symptomatic aortic stenosis is a Class I indication for surgical valve replacement according to the American Heart Association and American College of Cardiology guidelines for valvular heart disease.21
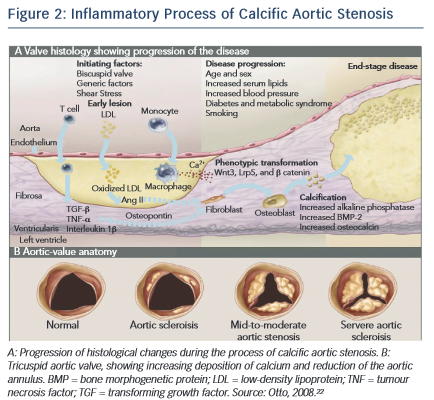
CAVD is currently considered as an actively regulated and progressive disease, characterised by a cascade of cellular changes that initially cause fibrotic thickening, followed by extensive calcification of the aortic valve leaflets. This in turn leads to significant aortic valve stenosis and eventual left ventricular outflow obstruction (see Figure 2 ),10,22 for which surgical replacement remains the only viable treatment option. Currently there is no approved pharmacological treatment to stop the progression of CAVD. 23 Descriptive studies using human specimens have demonstrated the hallmark features of this disease, including early atherosclerosis, cell proliferation and osteoblast expression. 24–26
CAVD and Traditional Risk Factors for Atherosclerosis
Aortic valve stenosis was first described by Lazare Riviere in 1663. 27 In the early 1900s, eminent pathologists such as Monckeberg, described CAVD as a passive degenerative process associated with rheumatic fever or aging, during which serum calcium attaches to the valve surface and binds to the leaflet to form nodules. 28
In more recent decades, several studies have implicated the traditional risk factors for cardiovascular atherosclerosis in the development of CAVD. Atherosclerosis is a complex and multifactorial process that produces a lesion composed of lipids, 29,30 macrophages, 31 proliferating smooth muscle cells 32 and apoptosis. 33 It is regulated by endothelial nitric oxide synthase, 34–38 and over time causes occlusion of the vessel diameter. Total cholesterol, increased low-density lipoprotein (LDL) cholesterol, increased lipoprotein(a), increased triglycerides, decreased high-density lipoprotein cholesterol, male sex, cigarette smoking, hypertension, and diabetes mellitus have been reported to increase the incidence of aortic stenosis, and are likely contribute to endothelial dysfunction and leaflet damage. 2,3,39–43 The presence of LDL and atherosclerosis in calcified valves in surgical pathological studies supports the hypothesis of a common cellular mechanism. 44,45 Furthermore, patients with familial hypercholesterolaemia develop aggressive peripheral vascular disease, coronary artery disease and aortic valve lesions, which calcify with age. 39,46–4
Oxidised LDL (oxLDL) is implicated in vascular calcification associated with atherosclerosis. 49,50 Elevated blood levels of oxLDL correlate with aortic valve calcification and fibrosis, 51 and oxLDL accumulation in calcific, stenotic aortic valves is well described. 52–56 Metabolic bone diseases – including Paget’s disease, secondary hyperparathyroidism and renal disease – as well as increased serum creatinine and calcium are also linked to progression of valve calcification, but include only a relative minority of patients who have aortic stenosis. 57–59 Understanding of these clinical risk factors provides the foundation for cellular studies and the potential for targeted medical therapies for this disease, similar to vascular atherosclerosis. However, the overall evidence indicated by the presence of atherosclerotic risk factors may partly explain why some patients who have congenitally abnormal valves develop aortic stenosis and require valve replacement sooner than those without risk factors. If atherosclerotic risk factors are important in the development of valvular heart disease, then experimental models of atherosclerosis are important in the understanding of this process. Studies in mice and rabbits have confirmed that experimental hypercholesterolaemia causes both atherosclerosis and calcification in the aortic valves. 60–64 Two months of cholesterol diet treatment in an experimental rabbit model induced marked thickening and complex calcification in the aortic valve leaflets. The model was extended to test the pharmacological effect of atorvastatin and angiotensin receptor antagonists on the inhibition of atherosclerosis pathways and calcification. 65–69 Other pathways, such as Wnt signalling and increased calcium concentration via kallikrein-kinin signalling, are involved in CAVD. Wnt proteins interact with trans-membrane receptors, in particular LDL receptors, and inhibit the effect of the degradation of the intracellular protein β catenin. In turn, β catenins mediate osteoblastic transformation of VICs and bone production. In vitro, atorvastatin – an inhibitor of LDL-cholesterol in blood – can neutralise this signal pathway in mice models. 66,70–72
The molecular and cellular processes that contribute to aortic valve stenosis are not fully characterised, but could provide insights into the development of new therapeutic approaches.
Heart valves comprise a heterogeneous population of valvular endothelial cells and VICs, which maintain valve homeostasis and structural leaflet integrity. VICs, the most abundant cell type in the heart valve, play a key role in CAVD progression. 73 Various VIC phenotypes have been identified in diseased human heart valves, 74 including quiescent fibroblast-like VICs, which upon pathological cues can differentiate into activated myofibroblast-like VICs; and osteoblast- like VICs, which are responsible for the active deposition of calcium in CAVD. 53,62,74 Additionally, several studies have demonstrated the ability of VICs to undergo osteogenic differentiation. 26,67,75
CAVD and Shear Stress
Although atherosclerotic coronary artery disease and CAVD share common features, they do have differences in rheology. This difference may provide at least a partial explanation for the differences in pathophysiology and response to therapy. 76–80 CAVD is characterised by pulsatile shear stress on the ventricular side and low and reciprocating shear stress on the aortic side, 81 whereas the coronary artery is exposed to sustained laminar blood flow under normal circumstances. 82 As stenosis progresses, wall shear stress across the aortic valve dramatically increases. 76 Ahamed and colleagues have demonstrated that in vitro shear stress can activate latent transforming growth factor (TGF)- β 1, 82 a critical pro-fibrotic growth factor that can induce fibrosis and calcification. 83 They also showed that active TGF- β 1 could be eluted from thrombi formed in response to vascular injury in the carotid artery of mice where partial occlusion may have led to high local shear stress. 82 Subsequently, Albro et al. independently confirmed that shear stress can activate latent TGF- β 1 in synovial fluid. 83 These data raise the possibility of an association between the activation of circulating latent TGF- β 1 under high shear stress and the development of CAVD. Because platelets contribute ~45 % of the baseline circulating TGF- β 1 level 84 and have 40–100 times more latent TGF- β 1 than any other cells, 85 it is possible that shear stress has two separate effects – inducing release of latent TGF- β 1 from platelets and activating the released latent TGF- β 1. This mechanism may contribute to the progression of CAVD, because aortic valve narrowing increases shear stress resulting in greater release of platelet TGF- β 1 and TGF- β 1 activation. This in turn may lead to progressive valve narrowing and fibrosis, and thus even greater shear stress.
Calcifying valves initially have macrophage and T-cell infiltrates as a result of endothelial injury. 74 Bone morphogenetic protein (BMP)-2 and BMP-4 are then expressed by myofibroblasts and preosteoblasts adjacent to these lymphocytic infiltrates. 74 Furthermore, cardiac valves express markers of osteoblastic differentiation, including core-binding factor alpha 1 and osteocalcin. 26 These valves also calcify in a manner similar to osteogenesis, with lamellar bone evident in the majority of pathological specimens examined. 85 Congenitally bicuspid aortic valves uniformly show signs of calcification by the time individuals reach age 30, 86 which may, in part, be attributable to the particular mechanical stressors to which these valves are subjected. 87 Recently, the molecular mechanism underlying bicuspid aortic valve calcification was solved. Mutations in the transcriptional regulator NOTCH1 resulted in aortic valve anomalies and severe calcification, owing to impaired repression of the osteoblast stimulator runt-related transcription factor 2 (RUNX2). 88
Recent evidence suggests that CAVD is the result of an active inflammatory process affecting the valve and leading to osteoblastic transformation with bone formation of VICs by activation of the receptor activator of nuclear factor- κ B (RANK). 89
Regulatory Pathways
There is increasing evidence that regulatory pathways that control heart valve development also are active with valve pathogenesis later in life. CAVD includes the activation of VICs in addition to increased expression of transcription factors that regulate the earliest events of valvulogenesis in the developing embryo. 90 In addition to valve developmental pathways, regulatory proteins that promote the development of cartilage and bone lineages also are active in diseased valves. 91 Thus, knowledge of the molecular regulatory pathways that control valve development will likely be informative in determining the molecular mechanisms of valve pathogenesis.
Aetiology
CAVD has multifactorial aetiology. Many factors are centered on an inflammatory process affecting the valve and leading to calcification, 74,85 including deposition of LDLs, 44,45 osteoblastic transformation with bone formation of valvular interstitial cells, connective tissue synthesis and tissue remodelling. On a microscopic level, the aortic leaflets contain disorganised collagen fibres, chronic inflammatory cells, extracellular bone matrix proteins, lipidic proteins and bone minerals. 5 Calcification of the valve occurs following trans-differentiation of the VICs through a myofibroblast stage and into osteoblast cells. 71,92
Half of adults undergoing aortic valve replacement have a bicuspid aortic valve associated, and nearly all of them will need to have a new valve inserted. 93 Shear stress occurring with each cardiac systole is greater in a bicuspid valve than in a tri leaflet structure and these valves calcify earlier. 93
Interestingly, the expression of RANK ligand (RANKL) by osteoblast cells will be actively involved in the activation and differentiation of osteoclast cells. 89 RANKL levels normally rise with age and can predict cardiovascular events in humans, while osteoprotegerin (a physiological inhibitor of RANK) deficit can lead to vascular calcification in animal models. 94,95 This study highlights an in vitro model to assess the mechanisms of aortic valve calcification.95
Molecular Mechanisms of Calcification
The processes of aortic valve stenosis and calcification share many similarities with atherosclerosis, and the pathologies of both conditions have similar risk factors and histopathology. 2 Activation of VICs and pathways of calcific aortic stenosis is the result of mechanical and shear stress, endothelial damage and deposition of LDLs, triggering inflammatory events and attracting inflammatory cells (monocytes, macrophages and T cells).
These cells produce cytokines, including TGF- β , which regulates cell proliferation and differentiation; tumour necrosis factor- α , whose primary function is the regulation of the immune cells; and interleukin 2, which is produced by activated T lymphocytes with growth factor activity.1
VICs activated by the inflammatory process are designated myofibroblasts.5 These cells will develop angiogenic activity and produce matrix metalloproteinases, proteins that are involved in tissue remodelling and support VIC activation and transformation.96,97 During this process activated VICs differentiate into osteoblasts.
In Vitro Studies
Initial studies in our laboratory have involved the establishment and validation of porcine VIC isolation, culture and calcification procedures and the effect of denosumab on in vitro calcification. During the characterisation of porcine VICs, the first objective was to determine the expression level of a common marker of myofibroblast phenotype, α -actin, to demonstrate that active VICs were present in the samples. The expression of RUNX2, a major regulator of osteoblast differentiation, was analysed to corroborate that CardiologyCardiology the effect of the complete transdifferentiation of VICs had taken place and that the osteoblast phenotype was present. Furthermore, changes in the expression of TGF- β (a promoter of osteogenesis), were detected and recorded. Additionally, RhoA, a regulator of nodule formation in myofibroblasts, was analysed, followed by examining changes in the expression of RANKL, a key regulator of bone metabolism. Finally, calponin, a protein with potential capability to inhibit bone formation, was measured to complete the genetic studies. TGF- β can increase calcium and collagen deposition.98 It is known that TGF- β can also stimulate the expression of RANK on pre-osteoclastic cells, and in this way increase osteoclastic sensitivity to RANKL.99 RANKL is expressed in the membrane of osteoblasts and monocytes. As yet there is still no evidence that TGF- β promotes calcification in porcine VICs by increasing RANK expression levels.
Our recent unpublished studies demonstrated the upregulation of key molecules during the spontaneous calcification of porcine VICs with an increase of calcium, collagen and alkaline phosphatase (ALP) activity. In vitro calcification was determined using standard staining and enzyme activity assays. Calcification in pig VICs was induced with sodium phosphate. The cells expressed markers for both vascular smooth muscle cells and osteoblasts, suggesting a transdifferentiation of the phenotype. Upregulation of α -actin, RUNX2, TGF- β and RhoA and downregulation of calponin were noted, with no changes seen in RANKL expression. Sodium phosphate increased nodular formation by day 7 and ALP activity of porcine VICs by day 14. The findings suggest that porcine VICs may be a good model to study the process of CAVD.100
Denosumab as a Potential Inhibitor of VIC Calcification In Vitro
Denosumab is a human IgG 2 monoclonal antibody designed to target RANKL,101 which is expressed on the membrane of the osteoblasts and osteoclasts. Denosumab is used in the treatment of osteoporosis. Additionally, owing to its mechanism that blocks the receptor RANKL, it neutralises the activation of RANK receptors on the membrane of pre-osteoclast cells.More research is needed to address the interaction between RANK receptor and denosumab in porcine VICs.
Our recent unpublished studies showed that 50 μ g/mL denosumab inhibited induced calcium deposition to basal levels in porcine VIC culture.100 Although associated with bone loss and shown to reduce vascular calcification, the effect of denosumab on calcification of human VICs is unknown. Recently, denosumab has been shown to reduce calcium deposition in the aorta, although the mechanisms by which it affects ectopic calcification are poorly understood.102 Furthermore, osteoprotegerin (a signalling protein receptor and a member of the tumour necrosis factor receptor family) has been shown to stop ectopic calcification in vitro via a similar mechanism to denosumab, but there is still not enough evidence of any effect in reverting the process of calcification. Osteprotegerin’s mechanism of action is to block RANKL-RANK receptor interaction. 94,95 A fuller understanding of the mechanisms of action of denosumab may identify a novel therapeutic approach for clinical treatment, supplementing the current surgical approach. It should be noted that extrapolation of the results obtained in an in vitro porcine model to humans should be cautious, as species variations are likely to exist. Although it is not possible to include all mechanisms involved in CAVD in a single model, experimental models can contribute towards identifying the role several factors may play in the development of CAVD.