Although the first study on biomarker-guided therapy of chronic heart failure (HF) was published 15 years ago,1 there is still no real proof of the superiority of this approach as compared with standard therapy. The guidelines of the European Society of Cardiology simply state that: “several RCTs that evaluated natriuretic peptide-guided treatment (intensifying treatment in order to lower peptide levels) have given conflicting results. It is uncertain whether outcome is better using this approach than by simply optimising treatment according to guidelines”. Apart from being markers of function of different organs, biomarkers, mainly natriuretic peptides, are considered useful only for excluding HF and as prognostic markers.2 However, several metaanalyses,3–6 including a very recent one based on individual patientdata (IPD),7 have found that natriuretic peptide-guided therapy may reduce both mortality and HF related hospitalisations. However, one recent systematic review was less positive and concluded that current evidence does not support the use of B-type naturiuretic peptide (BNP) and N-terminal pro (NT-pro)BNP. to guide HF therapy.8 This review addresses current evidence of natriuretic peptide-guided therapy and discusses potential reasons why this approach needs further investigation. Moreover, it will give short insight into other emerging biomarkers that are promising for therapy guidance in chronic HF.
Natriuretic Peptide Guided Heart Failure Therapy
Levels of natriuretic peptides, other than BNP and the N-terminal residual of its pro-hormone NT-proBNP, have little or no clinical role in HF, at least in Europe and the US. Other biomarkers have not yet been sufficiently studied in guiding chronic therapy. Therefore, this review focuses largely on these two peptides. In order to understand the concept of natriuretic peptide-guided therapy in chronic HF several aspects are important, as summarised in a recent review.9 Thus, HF is a very costly chronic disease (around 2 % of the total healthcare budget spent for HF) with increasing prevalence (at present there about 10 million HF patients in Europe).10 Prognosis remains poor despite significant advances in therapy11,12 and to some extent, this is related to the insufficient use of available treatment in HF.13,14 Achieving optimal therapy seems to be extremely difficult and additional markers to identify those patients in most need may be helpful. Natriuretic peptides may be very useful in this as they are strong prognostic markers across the whole spectrum of HF, particularly in the chronic stage15 and seem to change relatively little if patients are clinically stable.16 More importantly, natriuretic peptides change over time in parallel with change in prognosis17,18 and treatment of chronic HF influences plasma levels of natriuretic peptides. Thus, loop diuretics, angiotensin-converting enzyme (ACE)-inhibitors, angiotensin-II receptor blockers (ARB) and mineralocorticoid receptor antagonists (MRA), as well as cardiac resynchronisation therapy (CRT), may cause a fall in natriuretic peptide levels. The response to beta-blockade is more complex with a potential initial rise, but long-term fall.9 Taken together, natriuretic peptides may be very suitable for monitoring and guiding chronic therapy in HF.
Open original
Open in new tab
Open ppt
Clinical Trials
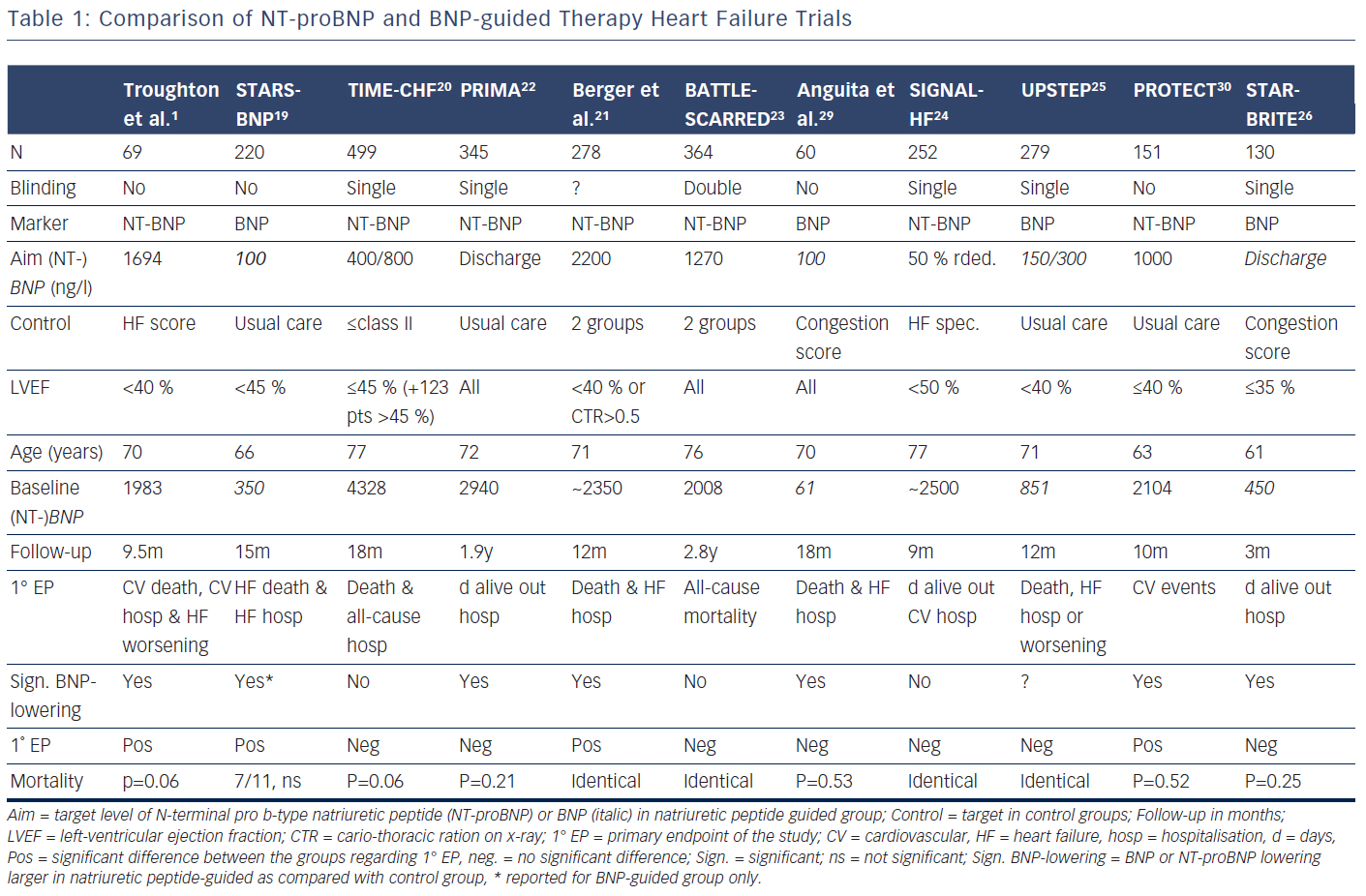
Several trials have investigated the concept that natriuretic peptide may help to optimise and up-titrate medical therapy in chronic HF (Tables 1 and 2).1,19–30 These studies included between 60 and 499 patients. The largest study additionally included 123 patients with preserved left-ventricular ejection fraction (LVEF),20,27,31 whereas in some of the other studies a smaller proportion of patients with preserved LVEF (HFpEF) were included in the main study without distinction based on LVEF (Table 1). In addition, a very small study of 41 patients investigated BNPguidance to up-titrate beta-blockade.32 A medium-sized single-blinded trial (n=407) investigated the value of increasing levels of NT-proBNP as an indicator for deterioration in patients with HF with reduced ejection fraction (HFrEF) already on optimised medical therapy.33 This latter study found no effect, but the intervention did not result in any changes in therapy. This suggests that measuring natriuretic peptide levels might be more helpful for up-titrating and optimising therapy in HF. More than one trial investigating natriuretic peptides use in monitoring HF patients already on optimal therapy is required before a clear answer can be given as to whether long-term monitoring using natriuretic peptides is of value. Effects of NT-proBNP guidance during 12 months’ optimising phase were maintained long-term even if NT-proBNP levels were no longer used to monitor patients in TIME-CHF28.
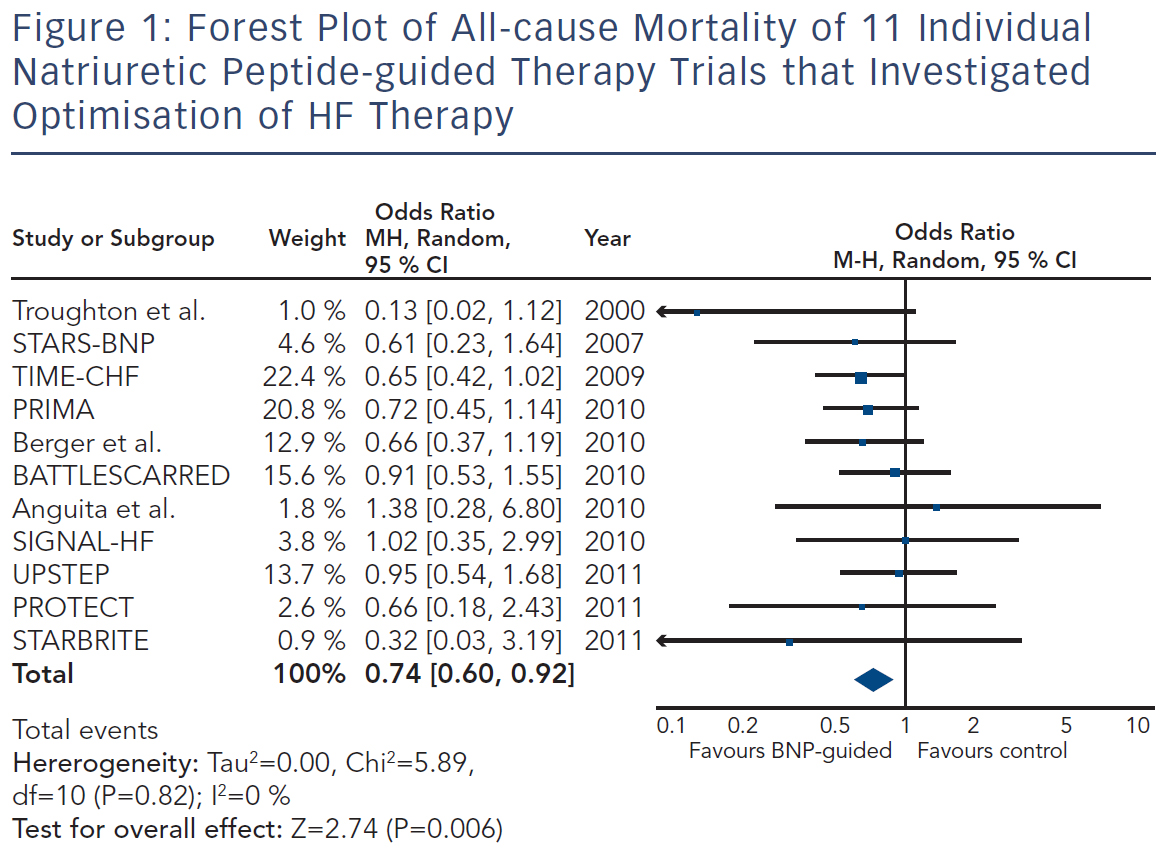
Because the limited number of patients included in these trials was insufficient to convincingly show beneficial effects of natriuretic peptideguided therapy, several meta-analyses were performed. Each showed positive effects on HF-related hospitalisation and mortality. Figure 1 depicts the forest plot of all-cause mortality of the 11 individual trials1,19–26,29,30 investigating optimising medication based on natriuretic peptide levels (Review Manager Version 5.3, Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration 2014). There was no significant heterogeneity between the trials, as was also found by all meta-analyses.
Is there any Interaction with Sub-groups?
A recent analysis based on IPD of eight of the trials1,19–24,27,30 showed different response to natriuretic peptide-guided therapy comparing HFrEF and HFpEF.34 This analysis also included the largest HFpEF population of all the studies,27 in contrast to all other meta-analyses. Whereas HFrEF patients defined as left-ventricular ejection fraction (LVEF)≤45 % on natriuretic peptide guided therapy had a reduced mortality with a hazard ratio (HR) of 0.78 (P=0.03), patients with HFpEF did not benefit at all (HR=1.22, P=0.41) with a strong interaction of the treatment response between the two groups (P<0.02).34 This difference comes as no surprise given that there is as yet no effective treatment for HFpEF, while the medication mentioned above – apart from diuretics – clearly improved outcome in HFrEF.2 Thus, intensifying ineffective treatment based on any means may not improve outcome. It has to be noted, however, that all the natriuretic peptide-guided trials were planned and conducted when this difference was largely unknown. Moreover in clinical practice, the same medication is still often used in HFpEF as it is recommended for HFrEF patients.35
The results from this IPD meta-analysis argues against such practice. Results from the natriuretic peptide-guided trials may provide important information on our understanding of treatment response in HF and the pathophysiology of the disease. In addition to the difference in the treatment response in HFpEF versus HFrEF, the potential effect of age is of interest. Some of the trials and the IPD-based meta-analysis reported significant differences in the effect of natriuretic peptide-guided therapy depending on age. Whereas a clearly significant positive effect was seen in patients aged under 75 years, no effect was seen in patients aged 75 and over.7,20,23 The recent additional analysis of the IPD shows that this difference may be explained by either the presence or absence of co-morbidities.34 Thus, more intensified HF therapy based on natriuretic peptide levels is less or not effective in the presence of significant co-morbidities. Two important questions arise from this finding. First, what are the reasons for this? Second, does this apply to HF therapy in general, i.e. is up-titration of medication in HF less efficacious in the presence of significant co-morbidities? Both questions cannot be easily answered.
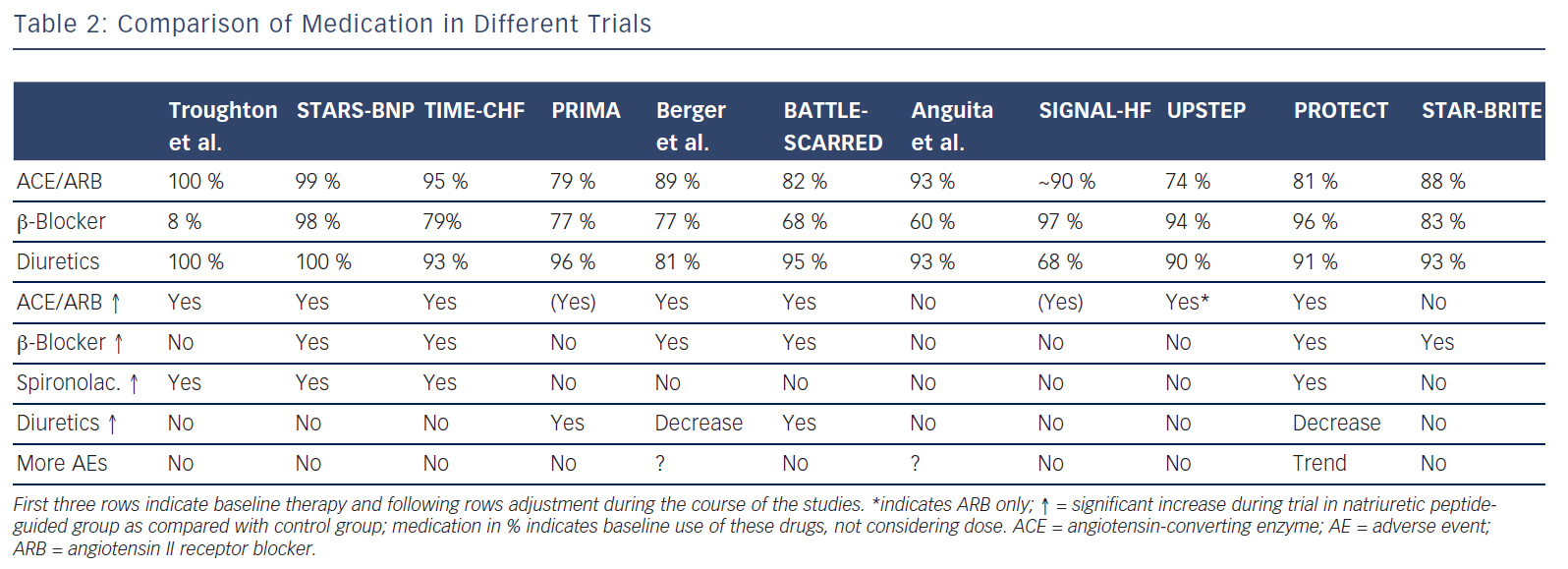
Briefly, co-morbidities might interfere with and change response to treatment, possibly resulting in reduced tolerance of [high doses of] medication. However, this has not yet been fully studied. Regarding natriuretic peptide-guided therapy, limited information suggests that natriuretic peptide guided-therapy and therefore more intensive treatment is safe and not accompanied by excessive side effects apart from mild hypotension36 (see Table 2). This also applied to worsening renal failure and hypokalaemia, although doses of loop diuretics and spironolactone were strongly related to these events, suggesting that up-titration of medication based on natriuretic peptides is safe when sufficient control is applied.37,38 Moreover, results show that natriuretic peptide-guided therapy may improve cardiac structure and function30,39 and that this effect is similarly present in those aged 75 years or older.40
Thus, the effects on HF itself in HFrEF may be irrespective of age and co-morbidities, but may be to some extent concealed by events related to co-morbidities. This is in line with the lack of interaction with age in the large randomised controlled treatment trials in HF2, as in these trials, patients with significant co-morbidities were largely excluded. Adequate trials are required to address this important question, in particular, prospective studies are urgently needed investigating the effects of treatment following current guidelines in the frail elderly with significant co-morbidities. This not only applies to natriuretic peptideguided therapy, but even more so to treatment in general.
Limitations of Natriuretic Peptide Guided Heart Failure Therapy
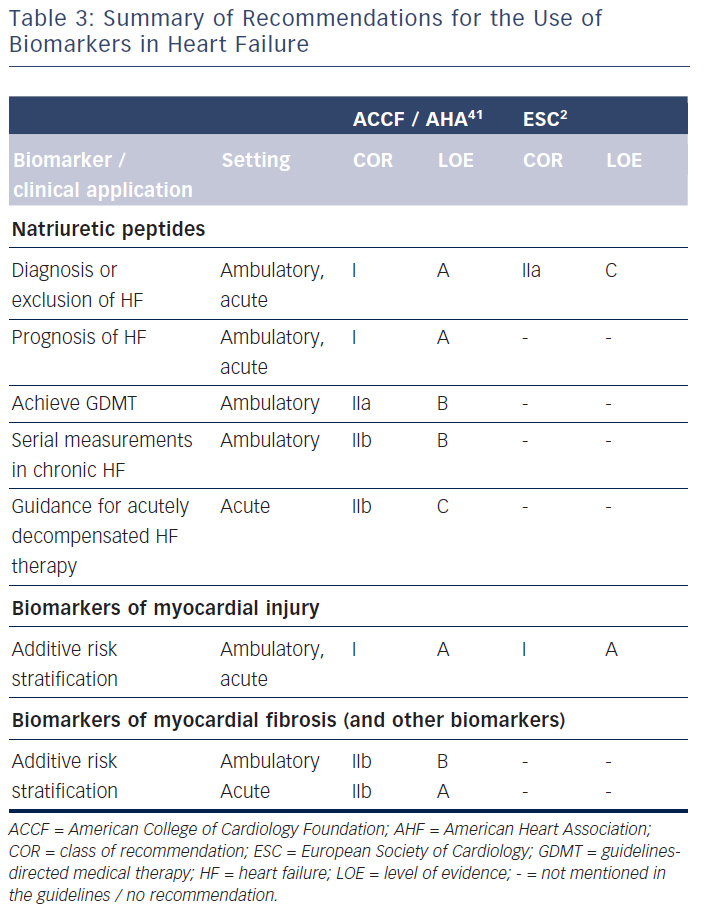
The question arises as to why natriuretic peptide-guided therapy in chronic HF – at least for HFrEF – is not yet recommended in the European guidelines2 despite the uniformly positive meta-analyses.3–7 It needs to be mentioned that the American guidelines interpret the results on natriuretic peptide-guided therapy rather differently.41 Thus, these guidelines encourage the use of BNP or NT-proBNP to achieve optimal dosing of guideline-directed medical therapy in clinically euvolaemic patients (class IIa recommendation).41 However, these guidelines also state that the usefulness of serial measurements of BNP or NT-proBNP to reduce hospitalisation or mortality in patients with HF is less well-established (class IIb recommendation).41 These two statements are contradictory and not easy to interpret for implementation into clinical practice. They are an expression of controversial opinions on this topic, as reflected in the lack of recommendations in the European guidelines.
Potentially there are several reasons for this. First, treatment response in the individual trials was not uniform; natriuretic peptideguided therapy resulted in a significant improvement of the primary endpoint in some, but not all studies (Table 1). This is partly related to the fact that different endpoints were used. For example, in TIMECHF, all-cause hospitalisation-free survival was the primary endpoint, which was not reduced and TIME-CHF – the largest natriuretic peptide-guided therapy study so far – is therefore considered as a negative study. However, if HF hospitalisation-free survival had been chosen as primary endpoint, as is often the case in HF trials, TIMECHF would have been a positive trial (HR=0.68, P=0.01).20 Second, the evidence is mainly based on relatively small trials; the largest included 499 HFrEF patients, which was formally negative as outlined above.20 Additionally, follow-up time varied significantly and was relatively short in some of the trials (Table 1). Third, natriuretic peptide-guided therapy trials did not investigate single-drug interventions (i.e. drug A versus placebo or drug B), but rather complex changes in the treatment, often left to the discretion of the treating physicians. Moreover, recommendations to react on persistently elevated natriuretic peptide levels differed considerably between trials as did the target values of natriuretic peptide levels (Table 1). Therefore, it is difficult to determine which aspects of natriuretic peptide-guided therapy gave the greatest contribution to improved outcome. It is not even entirely certain which trials have to be included in the meta-analyses; still, the results did not differ between them although they did not include exactly the same studies. In Figure 1, it is also obvious that the positive effect of natriuretic peptide-guided therapy is not based on one or two trials. This fact is also supported by sensitivity analysis of one of the meta-analyses.4 Importantly, trials that achieved greater intensification of drugs that showed beneficial effects on outcome in large treatment trials (i.e. ACE-inhibitors, ARBs, MRAs, beta-blockers), but not primarily intensified diuretic therapy, tended to have more positive effects on outcome than trials where mainly diuretic therapy was intensified or therapy was simply maintained (Tables 1 and 2).
Fourth, an often-heard argument against natriuretic peptide-guided therapy is the fact that natriuretic peptide levels were simply used to further optimise guideline-recommended therapy. Thus, applying guidelines correctly may be sufficient, without knowing natriuretic peptide levels. The results of the NorthStar study support this view.33 However, in the majority of patients, in daily practice treatment is far from being in accordance to the guidelines. In particular, optimal doses as recommended are often not achieved,35 but optimal doses are important to improve outcome.42 As stated in an editorial to the IPD meta-analysis,43 “use of disease-modifying therapy was much better than in typical practice”. This referred to the baseline use of medication in the natriuretic peptide-guided trials. Therefore, despite being better than in “real-world” practice even at baseline, knowing natriuretic peptide levels resulted in more aggressive up-titration and optimisation of therapy in chronic HF, thereby achieving better outcome. Simply applying guidelines to optimise therapy is more wishful thinking than clinical reality and additional means may help to achieve this goal. Disease management programmes may be helpful in this although results are not heterogeneous given the large differences between the various programmes.44 Repeatedly measuring natriuretic peptide levels is a simple means to optimise medical therapy in chronic HF and trials available so far suggest that such an approach is beneficial, even when directly comparing with disease management intervention.21
Finally, it is claimed that cost-effectiveness has not yet been proven for this approach. There are cost-effectiveness analyses of two of the trials of 777 patients and both concluded that NT-proBNP-guided therapy is highly cost-effective or even dominant (i.e. cost-saving and more efficient) as compared with standard care.45,46 Importantly, the additional measurements of NT-proBNP contributed to only a small fraction of the total costs, which were more than outweighed by reduction in HF-related hospitalisations.45 However, given the heterogeneity between the trials, interventions and populations, it remains to be determined as to whether cost-effectiveness is present for natriuretic peptide-guided therapy in general.
Taken together, there are important arguments in favour of the use of BNP or NT-proBNP levels for optimising the therapy in chronic HFrEF, at least in patients with few co-morbidities. This notion is also supported by most of the meta-analyses and/or systematic reviews,3–7,47 other than those which concluded that the evidence is of low quality.8 Despite these positive signals, major uncertainty remains to be resolved before a natriuretic peptide-guided treatment approach can be recommended as standard. A sufficiently large trial is required to address the remaining questions and, as a result, the US National Heart Lung Blood Institute has funded the GUIDing Evidence Based Therapy Using Biomarker Intensified Treatment (GUIDE-IT) study (NCT01685840, clinicaltrials.gov). GUIDE-IT is a prospective, multicentre, randomised trial of 1,100 patients with HFrEF at the time of discharge from an HF hospitalisation to either reduce NT-proBNP levels below 1,000 pg/ml or usual care.48 Enrolment began in January 2013 at more than 40 sites in the USA and Canada. In association with the data already reviewed above, GUIDE-IT should be adequately powered to provide more definitive answers about safety and efficacy of natriuretic peptide-guided therapy in chronic HF.
Natriuretic Peptides for Managing Other Conditions
A detailed overview of other conditions where natriuretic peptides have been shown to be beneficial is beyond the scope of this review, but a very short overview is provided. Both BNP and NT-proBNP have repeatedly been shown to be very useful in differential diagnosis of acute dyspnoea, both in the emergency department and in outpatient settings, mainly to exclude HF if levels are normal. This is also recognised in guidelines as class I indication.2,41 The prognostic value not only in chronic HF, but also in many other cardiac and non-cardiac conditions is beyond any doubt. However, knowing the prognosis does not necessarily mean knowing how to improve it, unless prospectively tested and this is only the case for chronic HF, as well as for more appropriate diagnosis in acute HF with faster and more relevant therapeutic intervention.49 Additionally, BNP levels may help in screening patients at risk for HF to aid earlier and more efficacious intervention with improved outcome, as shown in the STOP-HF trial.50 Additionally, the PONTIAC trial suggest that the diabetes patient with elevated NT-proBNP levels could profit from more intensive medical therapy (ACE-inhibitors and beta-blockers), also resulting in fewer HF events.51 These two studies show the potential of measuring natriuretic peptides in outpatient care beyond therapy guidance in chronic HF, but additional studies are needed to confirm the findings and to better define which patients profit most from natriuretic peptide measurements.
Biomarkers Other than Natriuretic Peptides
Biomarkers other than natriuretic peptides have not been prospectively tested to guide therapy in HF or to prevent HF. A huge number of biomarkers has shown to be of prognostic value, but few have clinical implications in cardiac diseases.52 In chronic HF, two biomarkers, soluble sST2 and galectin-3, have been particularly proposed as being useful in selection of patients most likely to benefit from specific interventions. Both fulfil requirements that make them attractive to guide therapy as already discussed above for natriuretic peptides. In particular, prognostic value of sST2 may be complementary to BNP/NT-proBNP,53 or possibly may be superior to BNP/NT-proBNP when serially measured.54 Moreover, they represent different pathophysiological pathways (sST2 from interleukin family, inflammation, fibrosis and remodelling; galactin-3 activates fibroblasts, fibrosis, inflammation) from natriuretic peptides and are largely independent of them, which might indicate that they can also independently add to clinical decision making. Importantly, both markers have shown some interaction with therapy response in HF. Thus, galectin-3 might predict lack of treatment response to statins in HF.55 However, similar potential interaction with MRA therapy response was not found in HF.56 A relationship between sST2 levels and the effect of eplerenone in post-MI patients with reduced LVEF was found,57 thus, sST2 predicted reverse remodelling, which could be attenuated in patients receiving eplerenone. Likewise, a high dose of beta-blockade was found to be particularly useful in patients with high sST2 levels.58 Although promising, it is important to note that these were all post-hoc analyses investigating only a single time-point of biomarker measurement and are, at best, hypothesis-generating. Prospective studies investigating specific interventions based on these biomarker levels must be done before they can be incorporated into clinical practice. Given the time required before natriuretic peptide-guided therapy in chronic HF may be implemented into clinical practice, there is still a long way to go with these new biomarkers, but it may be hoped that studies testing these new biomarkers are planned more cooperatively and on a larger scale at an early stage.
Conclusions
The clinical use of biomarkers in heart failure is summarised in Table 3. Natriuretic peptides, namely BNP and NT-proBNP, are wellestablished for diagnostic process of heart failure and prediction of disease prognosis, both acutely and chronically. They may also be helpful in screening patients at risk for heart failure. Importantly, natriuretic peptides may help to guide chronic heart failure therapy, but guidelines in Europe2 and the USA41 do not agree on the potential clinical implementation of such an approach. Many other biomarkers have shown to be of prognostic value, but it is not clear, apart from markers of myocardial injury in the acute setting, how this could translate into improved clinical decision making.59