Transcatheter aortic valve implantation (TAVI) has undergone tremendous technological advancements since the first successful implantation in 2002 for symptomatic severe aortic valve stenosis (AS).1 It is currently considered as the standard of care for severe AS patients who have high surgical risk or may be deemed unsuitable for surgery.2 Broadly, there are two main categories of transcatheter aortic valve prostheses: balloon-expandable (BE) and self-expandable (SE). Limited data exists as to whether there are significant differences in clinical outcomes with these two different prosthesis types. As newer prosthesis generations become available and the indications for TAVI expand to include additional patients and indications, such as those with intermediate surgical risk,3 valve-in-valve procedures4 and bicuspid AS,5 it has become relevant to have comparative efficacy and safety data of contemporary BE and SE prostheses. This evidence-based focused review aims to provide an overview of the valve characteristics and the factors influencing prosthesis selection, imbibing data from the literature on the clinical outcomes of BE and SE TAVI prostheses.
Transcatheter Prosthetic Valve Designs, Implantation Techniques and Access Routes
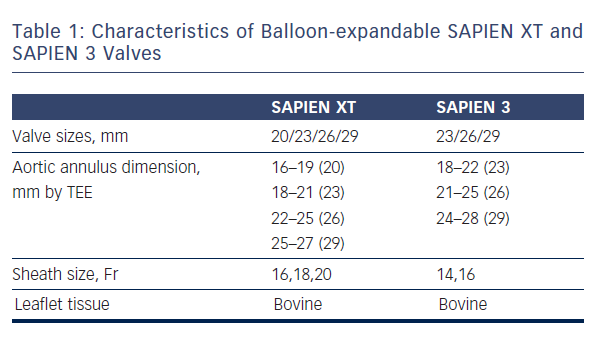
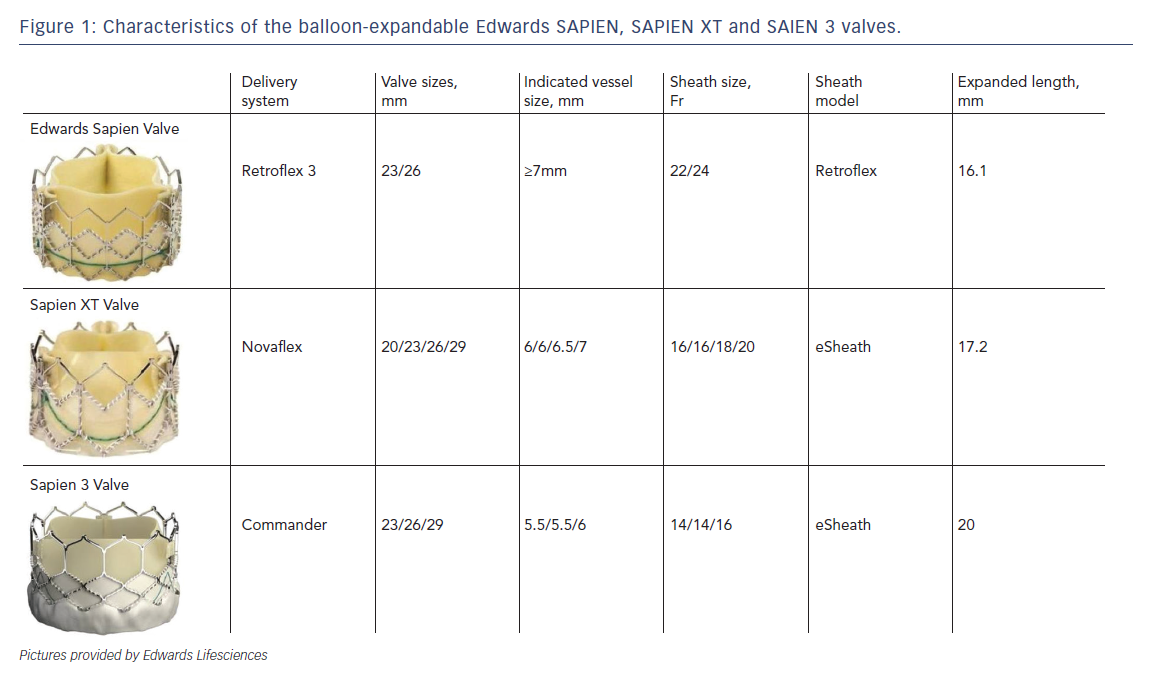
As mentioned earlier, most of the currently available prostheses employ either BE or SE technologies. Although these technologies are considered broadly comparable, differences exist and device characteristics play a role in prosthesis selection. A balloon-expandable aortic stent valve, consisting of a trileaflet bovine pericardial valve tissue mounted in a stainless steel frame, was the first BE valve prototype implanted in humans.1 Subsequent improvements of the valve and the delivery systems resulted in newer generations of BE prosthesis: Edwards SAPIEN, SAPIEN XT and SAPIEN 3 valves (Edwards Lifesciences, Irvine, CA) (see Table 1, Figure 1).6 The Edwards SAPIEN valve, which was available in 23/26 mm sizes requiring 22/24 Fr delivery catheters respectively, consisted of a tubular slotted stainless steel stent frame with unidirectional trileaflet bovine pericardial tissue and a fabric skirt made of polyethylene terephthalate that improved sealing. The SAPIEN XT valve consists of a trileaflet pericardial bovine valve mounted in a cobalt chromium stent frame having fewer rows, columns and vertical struts between commissures to reduce the valve profile. This valve is available in 20/23/26/29 mm sizes, and is implanted through 16 Fr (20/23 mm valves), 18 Fr (26 mm valve) or 20 Fr (29 mm valve) expandable sheaths. The SAPIEN 3 is the latest generation of the BE valves also having a trileaflet pericardial bovine valve mounted in a cobalt chromium stent, but incorporating an additional outer fabric skirt to further reduce paravalvular leak.
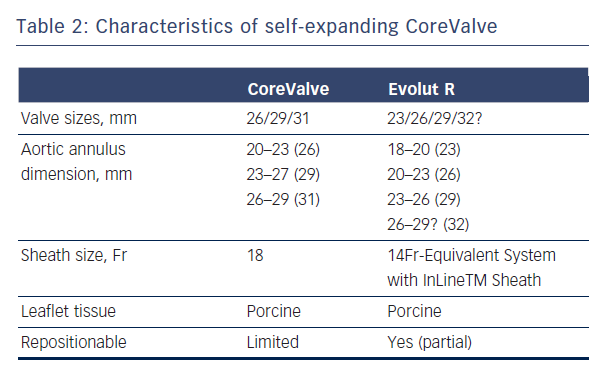
The CoreValve (Medtronic Inc; Minneapolis, MN), prototype of SE valves, is comprised of trileaflet porcine pericardial tissue sutured into a wire frame of nitinol, a nickel-titanium alloy that has temperature-associated shape memory features.7 The valve has three components: a high radial strength inflow segment that extends 4-6 mm below the annulus, a supra-annular segment containing the leaflet, and an outflow segment that aligns the stent with the aortic root.7 The first generation SE CoreValve was available in 26/29 mm inflow diameter sizes with an 18 Fr diameter delivery system. Current generation of CoreValve is available in 23/26/29/31 mm sizes and 18 Fr delivery systems (see Table 2, Figure 2).
A balloon aortic valvuloplasty is usually performed prior to both SE and BE valve implantation, though this step is commonly omitted in current clinical practice. The BE valve is implanted by positioning the valve under fluoroscopy, angiography ± transoesophageal echocardiography guidance with balloon inflation for valve expansion under rapid pacing, which minimises cardiac output in order to avoid
embolisation during deployment. The SE CoreValve is implanted by slow release of the valve at a marker band depth of 1-1.5 mm to obtain a final valve implantation depth of ~4 mm.7 The SE design allows partial repositionability with the newer generation devices (such as CoreValve Evolut R, Medtronic, Minneapolis, MN, Table 1) prior to final release, as opposed to BE technology where no repositioning is possible. The newer generation SE Lotus valve allows full repositionability prior to final implantation.
In the majority of patients, the retrograde transfemoral route is the preferred choice for implantation of both BE and SE prostheses.8 BE prostheses have also been delivered transapically, as well as through direct aortic access via minithoracotomy.9 The latter approaches are especially suitable in patients with severe peripheral artery disease and heavily calcified ascending aorta who are at risk of embolic complications. The SE CoreValve has also been delivered from the direct aortic in addition to the subclavian/axillary artery routes as an alternative in patients in whom the transfemoral route is contraindicated.10 The subclavian/transaxillary approach experience for BE valves is limited to a few cases.11,12
Clinical Outcomes
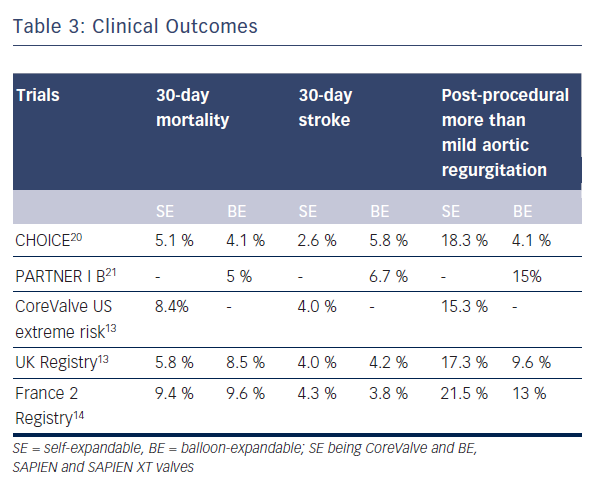
Data directly comparing SE and BE valves are limited and most of the data on clinical outcomes have been generated from registries and non-comparative randomised trials (see Table 3).13–18 Furthermore, there are no long-term comparative outcome studies. To date, the multicentre Comparison of Transcatheter Heart Valves in High Risk Patients with Severe AOrtIc Stenosis: Medtronic CoreValve versus Edwards SAPIEN XT (CHOICE) trial is the only randomised clinical trial directly comparing SE and BE valves (see Table 3).20
Mortality and Stroke Rate
Data from large multicentre registries and non-comparative randomised trials showed no difference in mortality between the 2Transcatheter Valves (PARTNER; cohort B) multicentre trial, which compared BE Edwards SAPIEN valve implantation with standard therapy including balloon aortic valvuloplasty, showed lesser mortality rate (43.4 % versus 68 %) and better functional class in the TAVI group at two years of follow-up.18,21–23 This trial was followed by the prospective single arm CoreValve Extreme Risk US Pivotal Trial study of SE CoreValve implantation, which reported a one-year all-cause mortality or major stroke rate of 26 %.19 Further data from randomised controlled trials of BE (PARTNER Cohort A trial) and SE (The CoreValve High Risk US Pivotal Trial) TAVI showed comparable and reduced mortality rates respectively as compared with surgical aortic valve replacement.18,22,24 Recent studies have shown decreased mortality rates with newer devices. In the SAPIEN 3 prospective study, transfemoral implantation of newer generation BE SAPIEN 3 valve resulted in a very low 30-day mortality of 2.1 % with no disabling strokes.25 Non-transfemoral access was associated with higher rates of mortality as well as stroke rates in this study. Finally, in the direct comparative CHOICE trial there was no significant difference in the 30-day mortality rates between the BE and SE groups (4.1 % for BE SAPIEN XT versus 5.1 % for SE CoreValve group).20
Similarly, comparative rates of embolic events were reported for SE and BE valves in several studies.27 In a large meta-analysis of neurological events after TAVI, 30-day event rates for CoreValve or SAPIEN prostheses were 3.1 % and 4.2 % respectively, the difference being non-significant.26 A statistically non-significant higher incidence of minor strokes was noted in the BE group in the CHOICE trial.20
The higher profile first generation BE SAPIEN prosthesis is likely to cause embolic events during stent positioning, whereas the lower profiled SE CoreValve may cause events mainly during stent deployment, due to shaving of calcific material from the native valve surface.28 Significantly more embolic events were observed during post-dilatation and repositioning with CoreValve as compared with BE valves.28 The unique advantage of resheathability and repositionability of modern SE valves may also translate into a higher liability to embolic events, though this remains hypothetical.
Profiles of both these devices have been improved to reduce stroke risk in newer generations, with the SE CoreValve Evolut R having incorporated an integrated sheathed delivery system and the BE SAPIEN 3 system with an atraumatic and smooth catheter profile with enhanced steerability.
Vascular Complications
The use of larger 22/24 F sheaths with the first generation BE SAPIEN valve was associated with a high rate of vascular complications as opposed to the smaller profile SE CoreValve.29 Recently, lower-profile BE valve systems such as the SAPIEN XT and SAPIEN 3 have become available with significant reduction in vascular complications. No significant difference between SE and BE groups with regard to vascular complications or bleeding was noted in the CHOICE trial (any vascular complication in 14 % for BE SAPIEN XT versus 12.8 % for SE CoreValve; p=0.78).20
Device Success
The composite endpoint of device success defined by the Valve Academic Research Consortium (VARC) evaluates acute device and procedural characteristics, and requires absence of prosthesis stenosis/regurgitation and correct implantation of only one valve for a successful procedure. In the CHOICE trial, the primary endpoint of device success was seen in 95.9 % of 121 patients treated with the BE device as compared with 77.5 % of 120 patients treated with the SE device (relative risk 1.24, 95 % CI 1.12–1.37).20 This difference in device success rates was largely driven by rates of more than mild valvular regurgitation, seen in 18.3 % of SE CoreValve patients versus 4.1 % of BE SAPIEN XT patients (p<0.001), and the more frequent need for implantation of a second valve in the SE CoreValve group, at 5.8 % versus 0.8 % (p=0.03).
Post-TAVI Aortic Regurgitation
Procedure-related incidence of aortic regurgitation, which includes transvalvular and paravalvular forms, is associated with increased short- and long-term mortality after TAVI and is a major limiting factor to its expanded use.18,19 Paravalvular aortic regurgitation results from poor annulus-prosthesis apposition and several factors, such as heavily calcified cusps, annulus-prosthesis mismatch and suboptimal prosthesis positioning, are thought to be responsible in both the SE and BE valve implantations. Transvalvular or central aortic regurgitation can be the result of leaflet restriction or damage during crimping or implantation as well as overdilatation of the valve.30
Some degree of aortic regurgitation, mostly mild, is observed in the majority of TAVI patients up to one-year follow-up. The incidence of more than mild aortic regurgitation after TAVI was found to be 7.4 % in a meta-analysis of post-TAVI outcomes.31 In another recent meta-analysis, the pooled estimate of more than mild post-TAVI aortic regurgitation was 11.7 % with a 95 % confidence interval of 9.6–14.1.32 In the UK TAVI registry, paravalvular regurgitation occurred more commonly in the SE CoreValve implants as compared with the BE SAPIEN implants (17.3 % versus 9.6 %).13 A similar finding was observed in the meta-analysis by Athappan et al.32 who reported more than mild aortic regurgitation to be more common with use of
the SE CoreValve as compared with the BE valves (16.0 % vs. 9.1 %, p = 0.005). In the landmark CHOICE trial, the incidence of more than mild aortic regurgitation by angiography was 4.1 % in the BE group as compared with 18.3 % in the SE group (p<0.001).20 In fact, the primary endpoint in this trial was mainly driven by a significantly lower frequency of more than mild aortic regurgitation as well as a lower need for an additional device in the BE group. Implantation of the BE SAPIEN 3 valve resulted in no severe aortic regurgitation at 30 days of follow-up in a recently published trial, and the rate of more than mild aortic regurgitation was as low as 3.5 %.25
Sherif et al.33 investigated anatomic predictors of paravalvular regurgitation after SE CoreValve implantation and found the angle of the left ventricular outflow tract (LVOT) to the ascending aorta (AA) as well as the device depth in relation to the noncoronary cusp to be independent predictors. Paravalvular regurgitation was more likely in patients with greater LVOT-AA angle as a result of the incomplete sealing of the paravalvular space. Too low as well as too high implantations also facilitated regurgitation via the uncovered struts and the space between prosthesis and annulus respectively. Despite high implantation and adequate sizing employed in the CHOICE trial, higher rate of regurgitation was observed in the CoreValve group, raising concerns regarding inadequate radial strength of SE devices, which can be a contributing factor for regurgitation. Long-term studies are necessary to chart the progression of paravalvular regurgitation with time, since geometric remodelling of the annulus with a reduction in the degree of regurgitation may occur over time. Such reductions in severity of regurgitation may be of relevance for SE prostheses.
Need for New Pacemaker
The risk factors for development of high-grade heart block requiring permanent pacemaker in observational studies include use of SE valve and pre-existing right bundle branch block.34 In patients receiving the SE valve, the risk of requiring a permanent pacemaker can be as high as 42.5 % whereas the risk of this complication for BE valve is similar to that observed after balloon valvuloplasty or surgical valve replacement in clinical trials.35 Similar results were reproduced in the CHOICE trial, where 37.6 % of SE patients required a new pacemaker as compared to 17.3 % in the BE group.20 Higher incidence of significant heart block in SE valve patients could be explained by the interaction of its nitinol frame with the interventricular septum. Radial forces of the CoreValve stent are greater on the LVOT, especially if the LVOT diameter is small or tissue stiffness is high. Excessive radial force, especially in low implants, may contribute to heart block.36 Furthermore, SE CoreValve implantation is associated with a higher incidence of new left bundle branch block when compared to BE valve implantation and surgical valve replacement.37 Newer generation repositionable SE devices may help in reducing the pacemaker rate with a more targeted implantation.
Rare Complications
Occlusion of coronary artery ostia is a rare but life-threatening cause of ischaemia and hypotension following TAVI, with the majority of such complications reported after BE valve implantation. A recent large series reported an incidence of 0.81 % for BE versus 0.34 % for SE valves (p=0.02).38 Factors predisposing to coronary artery occlusion include small aortic sinuses, aortic annulus to coronary ostium distance less than 1 cm and significant asymmetric valve calcification. This complication was also reported to be more frequent in women and in patients with prior surgical bioprosthesis. In the randomised CHOICE trial, two patients belonging to the BE group had coronary obstruction as opposed to none in the SE group.20 Annulus rupture is another rare life-threatening complication and risk factors include BE valve implantation, small annular size, bulky calcification and aggressive predilatation.39,40 The reported incidences of valve embolism, valve malpositioning and need for more than one valve implantation are higher with SE valves.41 Implantation of ≥ 2 valves was higher in the SE group in the CHOICE trial (5.8 % for SE versus 0.8 % for BE).20 However, nearly half of BE valve embolisations occur towards the left ventricle as opposed to SE valves, which usually require surgery and are associated with a high mortality rate.4 BE valve embolisations are generally caused by anatomic and technical factors, which may be avoided with procedural planning. In recent years, a few cases of valve thrombosis with both BE and SE valves have been reported.
Valve Durability
Valve durability is important for the expansion of TAVI to younger patients, and both prosthesis types have been found to be durable in short- and intermediate-term studies. Toggweiler et al.42 evaluated the long-term haemodynamic changes of BE valves at five years’ follow-up and observed a decrease in mean aortic valve area of 0.06 cm 2/year and an increase in mean transvalvular gradient of 0.27 mmHg/year with no cases of structural valve failure. Similarly, the CoreValve CE Pivotal Study demonstrated excellent durability at four years in patients who were treated with the SE CoreValve System.7
Differential Indications for TAVI
In the early days of TAVI, prosthesis selection was largely determined by the size of the aortic annulus and that of the iliofemoral arteries, with the SE CoreValve being favoured in patients with larger aortic annulus and smaller iliofemoral arteries. In recent times, annulus morphology has influenced valve selection and SE CoreValve, thought to have the ability to adapt to various annular and root anatomies, is preferred by some operators for patients with an asymmetric aortic annulus on multislice computed tomography. Operator experience with regard to volume of cases being performed and familiarity with the device is likely to play a significant role in prosthesis selection, with low-volume centres more likely to use only one device type for all patients. Nevertheless, availability of a wide size array of newer generation BE and SE prostheses have made TAVI feasible for a wide range of patients with complex anatomies.
Traditionally, bicuspid AS is considered as a contraindication for TAVI because of the increased risk of associated adverse aortic events such as progressive aortic dilatation with device migration, aortic dissections, and significant paravalvular regurgitation owing to more frequent elliptical shape and asymmetric annulus. As a consequence of technological improvements and experience, TAVI has been increasingly performed with reasonable success in selected
patients with bicuspid aortic valve stenosis. Because of the potential complications mentioned above, these patients may be favoured to receive a repositionable SE valve prosthesis. Patients with minimal aortic valve calcification and predominant regurgitation are also likely to benefit from SE valve prostheses.
Finally, SE valves may have a beneficial role in patients undergoing valve-in-valve TAVI for small degenerated aortic bioprosthetic valves, where fewer gradients are achieved due to supra-annular valve function. On the other hand, BE valves could be used for valve-in-valve implantations for all four valve positions, namely tricuspid, pulmonary, mitral and aortic, whereas the SE CoreValve could only be used in the aortic position.
Newer Generation Devices
Since the first TAVI in 2002, a reasonable number of randomised trials and registries exist that provide valuable insights as to what is an optimal SE or BE prosthesis. An ideal BE prosthesis is one that has a low profile and allows optimal and accurate positioning within the native aortic valve and annulus with minimal risk of complications. An ideal SE valve is one that should be completely retrievable to allow precise device positioning, has a design that permits coronary artery access as well as optimal radial strength and ability to adapt to various annulus and root anatomies to provide adequate seal.
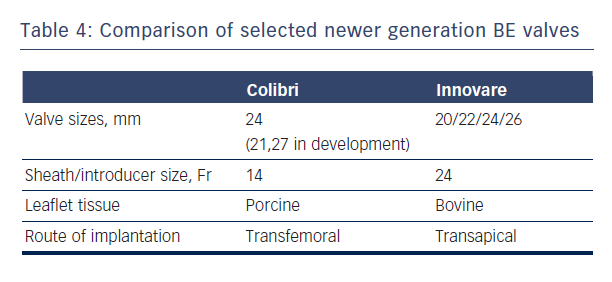
New generations of SE and BE devices address many of the major deficiencies of the first generation valves in terms of accuracy of positioning, repositionability, ease of use and enhanced features to minimise paravalvular leakage. Newer BE prostheses such as SAPIEN 3 and Colibri (Colibri Heart Valve, Broomfield, USA) and newer SE valves such as Evolute R and Lotus hold some promise in this regard.6
The Colibri valve is a low-profile (14-Fr) pre-crimped, and pre-packaged ready-for-use BE valve made of three independent pieces of porcine pericardium, which are created using a unique folding method and sewn into a stainless steel laser-cut frame. Another newer BE valve is the Innovare valve (Braile Biomedical, Brazil), which consists of a trileaflet bovine pericardial valve mounted in a cobalt-chromium alloy, available in sizes of 20/22/24/26 mm (see Table 4).43
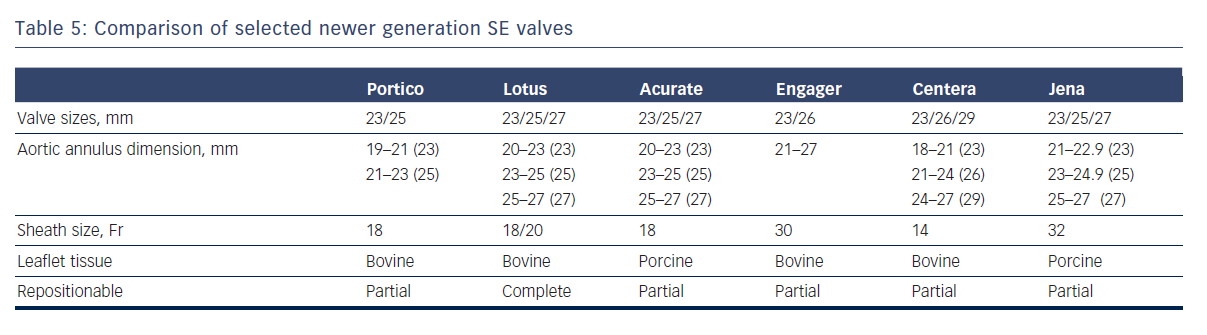
Newer SE and mechanical valves such as Lotus (Boston Scientific, Natick, MA), Acurate (Symetis SA, Ecublens, Switzerland), Portico (St. Jude Medical, St Paul, MN), Centera (Edwards Lifesciences, Irvine, CA), Engager (Medtronic Minneapolis,MN) and JenaValve (JenaValve Technology GmbH, Munich, Germany) aim to address potential limitations of earlier SE devices, such as lack of repositionability and ease of use, and offer important advantages (see Table 5).44–48 For instance, the non-flared inflow end of the Portico valve permits higher implantation depth with minimal left ventricular outflow tract protrusion, thereby reducing the risk of heart block.45 Because the valve leaflets are fully functional in the partially deployed state, extent of paravalvular regurgitation can be assessed prior to full release. The next generation Lotus valve provides a high radial strength and is also capable of being completely retrieved if necessary.44 It has an adaptive seal at the inflow portion that serves to improve apposition to the native annulus and reduce paravalvular regurgitation.
Finally, the Direct Flow (Direct Flow Medical, Santa Rosa, CA) is
a new distinctive prosthesis having a non-metallic valve frame and a flexible low-profile delivery system that allows repositionability and repeated assessment of full haemodynamic performance before final implantation. It consists of a bovine pericardial tissue valve that is mounted between two inflatable polyester rings and has the ability to adapt to the native aortic annulus and the left-ventricular outflow tract.49
Conclusion
In conclusion, a wide array of devices is currently available falling into the broad category of SE or BE valves, with a shift of the factors that dictate prosthesis selection – sheath and device dimensions in the early days to factors such as frame design, radial force and the anatomical characteristics of the annulus and root in the present era. Results from the CHOICE trial have provided some insights into short-term outcomes after SE and BE valve implantations; but as newer devices become available and indications for TAVI expand, long-term outcome comparative trials are needed to conclusively dictate device selection. Recently-launched technologies and devices are in their initial phases, and as more scientific data focusing on individual patient characteristics become available, indications for TAVI may expand to intermediate-risk and other population subsets of aortic valve disease.