Transcatheter aortic valve implantation (TAVI) is an effective treatment for patients with severe aortic valve stenosis and high operative risk.1 Despite its widespread adoption across the world, this procedure is still associated with several complications.2 Many of these, because their relatively high frequency (conduction disturbances, vascular complications, cerebrovascular events, paravalvular regurgitation, etc.), have been a field of deeper research aimed at defining potential predictive factors, clinical sequelae and procedural management.3–5 Nonetheless, TAVI has also been associated with very rare but life-threatening complications, such as coronary ostia obstruction and aortic rupture.6,7 Because of their extremely poor prognosis, prevention of these complications is of particular interest.
In this scenario, prosthesis type and size selection is part of the patient selection process that allows the operator to prevent these complications and finally determine procedural outcome.7,8 In this review, the techniques used either during pre-TAVI screening or during the procedure itself to avoid coronary occlusion and aortic rupture will be discussed
Coronary Occlusion
The occurrence of coronary occlusion after TAVI was first described in 2006.9 In contemporary series its incidence has usually been less than 1 %, ranging from 0 % to 4.1 %.2 A recent systematic review showed that reported cases of coronary obstruction following TAVI occurred more frequently in women and patients receiving a balloon-expandable transcatheter heart valve (THV) (SAPIEN, Edwards Lifesciences, Irvine, CA, US), and the left coronary artery was the most commonly involved.6
In native aortic valves, coronary ostia obstruction is related to two main procedural factors: 1) In the vast majority of cases it occurs due to the displacement of a bulky calcified native valve over a coronary ostium; 2) the second option is more hypothetical: it occurs following an obstruction by a portion of the THV frame or the sealing cuff placed directly over a coronary ostium; however, no cases of coronary obstruction related to the struts of the THV frame or to the cuff/leaflets of the transcatheter valve itself have been reported to date.
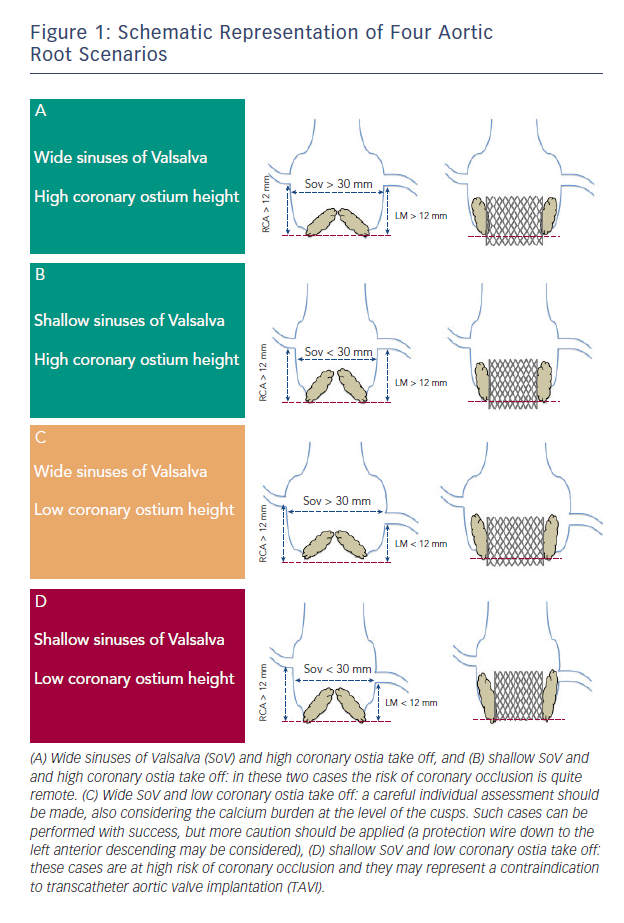
In both cases there are some anatomic features (narrow sinus of Valsalva, bulky leaflet calcifications, low-lying coronary ostia) that have been involved in its pathogenesis (see Figure 1). In a multicentre study enrolling 44 patients who suffered symptomatic coronary occlusion among a large series of 6,688 TAVIs, Ribeiro and colleagues demonstrated that lower-lying coronary ostium (<12 mm) and shallow sinuses of Valsalva (<30 mm) were the strongest anatomic factors associated with coronary obstruction.8 Nevertheless, the risk of coronary occlusion remains difficult to assess, and no definite criteria exist to exclude patients deemed at high risk. However, this study suggests that greater caution should be applied when we are dealing with patients candidate to TAVI having such anatomical features.
A careful evaluation by means of multimodality imaging can help to highline the bulkiness of the native cusps, the height of the coronary ostia and the dimensions of the sinus of Valsalva.
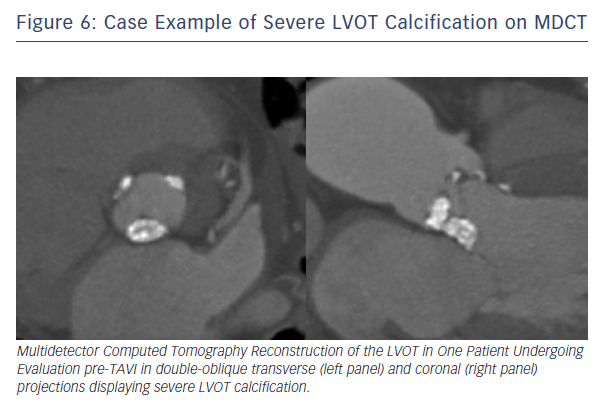
In daily clinical practice, these evaluations can be made by using multidetector computed tomography (MDCT), which allows for a granular 3D reconstruction of the aortic root, providing extremely reliable assessment of dimensions and calcium distribution (see Figure 2).10,11 3D echocardiogram is also an important tool for aortic root measurements and for pre-procedural assessment of the coronary ostia, but it is limited by its inability of characterise aortic root calcifications.12
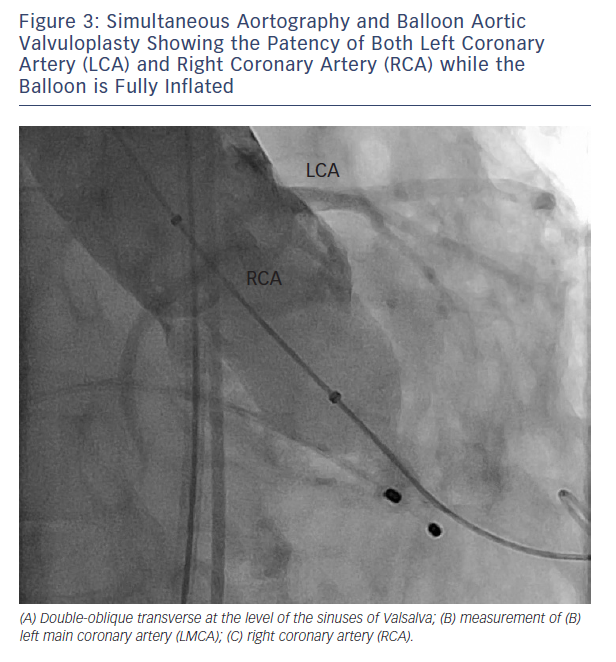
When coronary obstruction does occur, clinical presentation is generally characterise by severe hypotension, ST-segment changes and procedural ventricular arrhythmias.6,8 Successful management may require temporary cardiopulmonary support and revascularisation. When the MDCT assessment shows that a certain aortic root anatomy may be prone to develop coronary impairment after valve deployment, a pre-implant balloon valvuloplasty with simultaneous associated aortography may be useful to ensure the patency of the coronary ostia during balloon inflation; according to this technique, when the balloon is fully inflated, the coronary ostium could be occluded by the calcified cusps crushed against the wall of the sinuses of Valsalva (see Figure 3). In that case, the operator could consider terminating the procedure and considering other options.13 Alternatively, few strategies could be adopted: 1) implanting the prosthesis slightly lower into the left ventricle outflow tract (LVOT), in order to reduce the movement of the cusps toward the coronary ostia and the sinus walls (when the CoreValve prosthesis [Medtronic Inc., Minneapolis, MN, US] is implanted); 2) placing a coronary wire in the periphery of the left anterior descending in order to be ready to restore the patency of the coronary ostia by ballooning and/or stenting the left main, if it should be needed.6 If the patency of the coronary cannot be restored and the haemodynamic is poor, the valve should immediately be snared (CoreValve), or removed from its anatomical position by using an oversized balloon (i.e. SAPIEN prosthesis) and pulled up out into the ascending aorta to allow coronary perfusion to be re-established.14 Recently, Ribeiro and co-workers showed that PCI was the preferred strategy for the treatment of coronary obstruction following TAVI.8
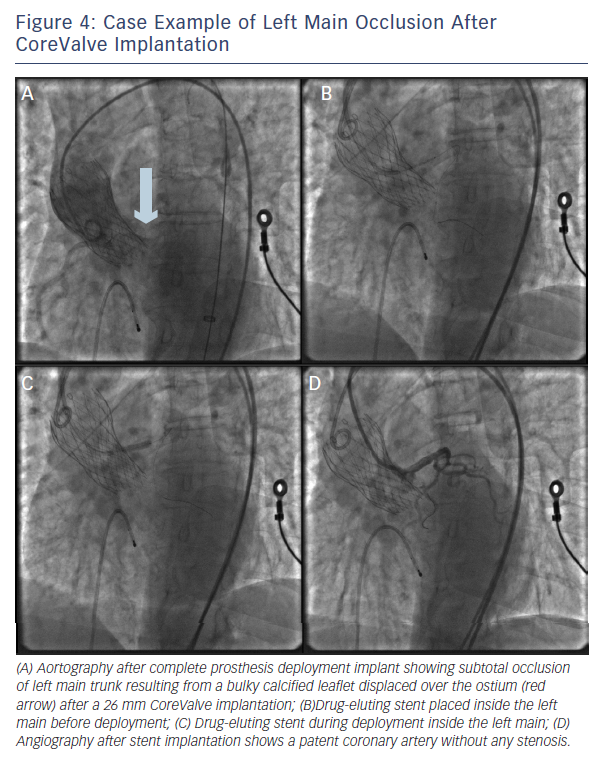
Importantly, PCI was feasible (attempted in 75 % of the patients) and had a success rate of 81.8 % (see Figure 4). Still, urgent coronary artery bypass graft (CABG) or mechanical haemodynamic support were needed in 14 % and 36 % of the patients, respectively.8
Valve-in-Valve
Coronary obstruction is three- to fourfold more common after TAVI in degenerated surgical bioprostheses compared with native valve TAVI.15 Recently, Dvir and colleagues reported a coronary obstruction incidence of 3.5 % of patients.15 The main predisposing factor in the setting of aortic valve-in-valve procedures is the proximity of the coronary ostia to the anticipated final position of the displaced bioprosthetic leaflets after THV implantation. Therefore, predisposing factors for coronary obstruction may include a supra-annular bioprosthetic valve, a narrow and low-lying sinotubular junction, bulky bioprosthetic leaflets, low-lying coronaries in narrow
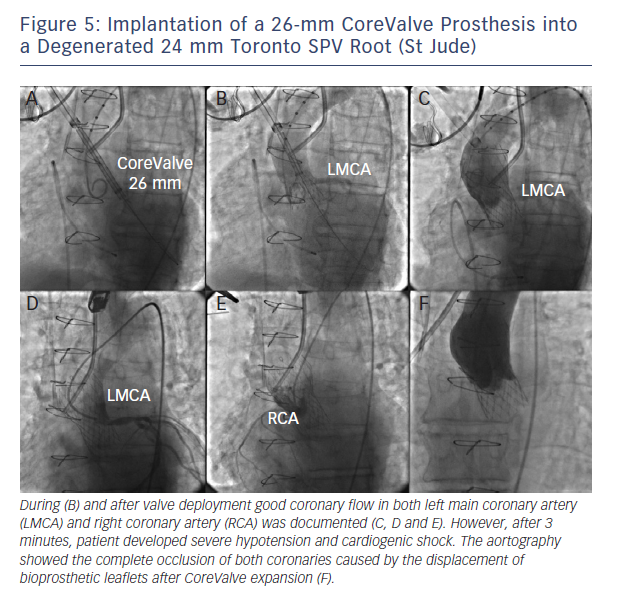
aortic root and reimplanted coronaries.15 Bearing these concepts in mind, it appears logical that stentless bioprosthetic valves or those that are internally stented (eg, Mitroflow, Sorin; Trifecta, St Jude Medical) may be at a higher risk because the leaflets of these bioprostheses may extend outward in a tubular fashion after valve implantation beyond the surgical device frame (see Figure 5).15,16 In this setting, meticulous fluoroscopic and cardiac MDCT assessment may identify most cases at risk.16
Aortic Root Rupture
Aortic root rupture after TAVI shares several aspects with coronary occlusion: 1) the frequency: a recent meta-analysis and a multicentre study reported a cumulative LVOT and annulus rupture rates of 1.1 % and 0.9 %, respectively;2,7 2) this complication is a particular concern with balloon-expandable prostheses because of the significant force applied during balloon deployment;7,17 3) clinical presentation, which can be dramatic: patients experiencing aortic root rupture (particularly those with uncontained rupture) develop sudden haemodynamic compromise and cardiogenic shock often unresponsive to inotropes.7
In such cases, median sternotomy and conversion into conventional aortic valve replacement usually represents the sole resort. Nevertheless, uncontained aortic root rupture is burdened by a very high mortality rate (75 % in our previous series). Differently, periaortic haematoma (also named ‘contained aortic root rupture’) carries a better prognosis, even though it belongs to the spectrum of the same pathology.7,17 Indeed, among 11 cases of periaortic haematoma reported in the same study, all patients were alive at 30 days.7
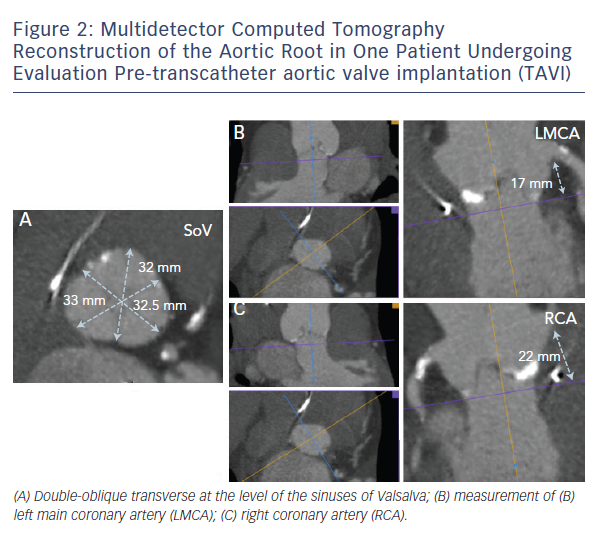
The question is: is it possible to predict aortic rupture and to apply any strategy to prevent it? In this context, 3D MDCT has been shown to provide a deeper understanding of aortic annular geometry and allow for prediction of aortic rupture.10 Recently, we showed that there are at least two important features associated with annular rupture/periaortic haematoma: 1) moderate or severe LVOT/subannular calcification (see Figure 6), and 2) significantly oversized prostheses (≥20 % area oversizing).7 Calcification in the LVOT is a particular concern in the context of TAVI, because this is a rigid, thin-walled structure. Therefore, detailed assessment of the distribution of calcium within the aortic root and LVOT may provide important additional information regarding the risk of aortic root injury. A recent observation suggests high LVOT/subannular region it is calcium located below the non-coronary cusp that is most predictive of aortic root injury.18 The reason for this finding is not entirely clear. Previous observations have suggested that the left aortic sinus may be the most vulnerable area with regard to aortic root injury possibly due to lack of supporting cardiac structures in this area.17,19 However, it may be speculated whether the culprit site is located at the site of calcification or at the aortic wall opposite to the calcification due to THV migration away from hard calcified areas during balloon expansion.
Historically, it has been shown that a certain degree of annular oversizing (5–1 5 % by area) is essential to mitigate the risk of significant paravalvular regurgitation with balloon-expandable valves.20 However, greater oversizing (>20 % by area) is indicated in borderline or transitional annulus, considering that available balloon-expandable valves have been available in 3 mm diameter increments, with nominal expanded diameters of 20, 23, 26 and 29 mm.21 In such situations (particularly when significant LVOT calcification, shallow sinuses of Valsalva or highly calcified aortic cusps are present), it may be safer to proceed with self-expanding (CoreValve, etc.) or inflatable (Direct Flow) TAVI platforms, which are rarely associated with annular rupture, when post-dilation is not performed. Alternatively, a recent approach of intentionally underexpanding balloon-expandable THVs by underfilling the deployment balloon has been proposed;21 this technique produced predictable reduction of THV expansion without adversely affecting procedural or short-term clinical or echocardiographic outcomes.21
Finally, in those cases of incomplete THV expansion causing residual paravalvular regurgitation, greater caution should be taken when performing balloon postdilatation in patients with significant LVOT calcification or shallow sinuses of Valsalva. This important factor will be likely addressed with the introduction of the new generation of balloon-expandable THV (SAPIEN 3, Edwards Lifesciences, Irvine, CA). In fact, it was recently demonstrated that a lesser degree of MDCT area oversizing might be employed for this new balloon-expandable THV.22
Conclusions
Coronary occlusion and rupture of the aortic root or annulus remain two major concerns of transcatheter aortic valve implantation technique. Despite their relatively low frequency they raised the interest of the scientific community as they carry an extremely poor prognosis. Prosthesis type and size selection is part of the patient selection process that allows the operator to prevent these complications. n