Orthotopic heart transplantation (OHT) is the most effective long-term therapy for end-stage heart disease, with implanted left ventricular assist devices (‘destination therapy’) as an alternative for selected patients. The denervation of the transplanted heart with complete loss of autonomic nervous system modulation, the use of immunosuppressant drugs, as well as the risk of allograft rejection (AR) and vasculopathy, all change the incidence, prognosis and treatment of tachyarrhythmias and bradyarrhythmias, as well as the mechanisms of sudden cardiac death (SCD).
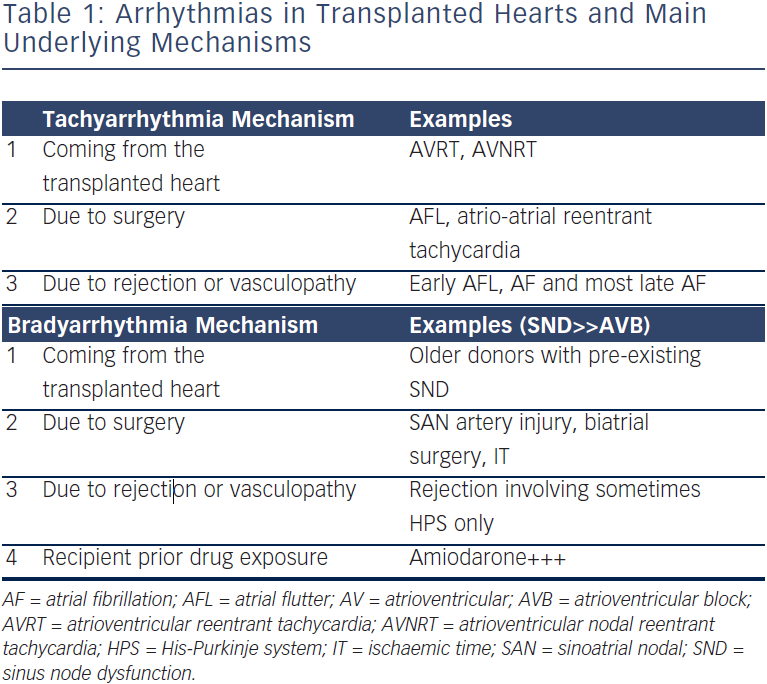
Arrhythmias post-OHT can be classified according to their underlying mechanisms (see Table 1). Tachyarrhythmias and bradyarrythmias can come from the transplanted heart, be due to the surgery itself or result from AR or vasculopathy. In addition, bradyarrhythmias may be caused by drugs taken by the recipient patient before surgery. This review aims to present the most common causes of arrhythmias in OHT patients, and to highlight the importance of ruling out and treating AR and vasculopathy – transplant coronary artery disease (TCAD), which should always be the first concern. The causative mechanisms represent different risk factors, and overlap is possible, which may increase the occurrence of such arrhythmias.
With the increasing success of radiofrequency (RF) ablation techniques, it is important that cardiologists are familiar with tachycardias and bradycardias in this context, which may benefit from a multidisciplinary approach in the management of the patient starting with the underlying mechanisms.
Tachyarrhythmias
Supraventricular Tachycardia
The most common atrial arrhythmia in OHT patients is atrial flutter (AFL) followed by atrial fibrillation (AF) with respective incidences of 2.8 %1 to 30.0 %2 and 0.3 %1 to 24.0 %.2 The discrepancies mostly reflect the fact that those studies have variable follow-up periods and often small cohorts. After 1991, the year of the first report of bicaval OHT, this technique progressively supplanted the prior standard atrial anastomosis OHT. In a small cohort of 66 patients, Grant et al.3 found a significant decrease in atrial arrhythmias in the post-operative period among patients undergoing bicaval OHT (9.7 %) compared with those undergoing standard OHT (37.1 %). Maintenance of atrial conformation with better ventricular filling and a reduced tendency to mitral and tricuspid regurgitation may have contributed to this acute decrease.4 In stable OHT patients, only reentrant atrial tachycardia (AT) linked to surgical scars at atrial suture lines seem decreased withbicaval OHT, but not other ATs.5 The major limitation of such studies is that this group also represents the most recently treated patients, and other factors including advances in medical therapy may contribute to the findings.
The largest and most recent studies report a smaller incidence of supraventricular tachycardias (SVTs) due to improvements in graft preservation, surgical techniques resulting in neural decentralisation and isolation of the posterior left atrial wall in addition to advances in immunosuppressant therapy. Thus, post-operative OHT AF or AFL (POAF, POAFL) is not a common finding compared with other thoracic surgeries. Indeed, Khan et al.1 first compared the incidence of AF, AFL and other SVTs in heart transplant versus matched low-risk coronary artery bypass graft (CABG) patients. They demonstrated that the OHT group had uncommon AT (0.3 % AF + 2.8 % AFL) when compared with CABG (25 % AF + 17 % AFL), and interestingly, ‘other SVT’ cases were not so different between two groups (1.3 versus 4.3 %, respectively). Dizon et al.6 compared the incidence of AF and AFL in heart transplant versus double-lung transplant and CABG surgery patients. Consistently, the OHT group had uncommon AT (4.6 % AF + 2.9 % AFL) when compared with the two other thoracic surgeries (18.9 % AF + 7.4 % AFL and 19.8 % AF + 3.8 % AFL, respectively). In a similar study, Noheria et al.7 also found that POAF occurred only in 6.5 % of OHT patients, and was much more frequent after maze (22.7 %) or CBAG (16.4 %) surgery. POAF is usually benign and thought to be secondary to the immediate post-operative inflammatory state.8 The two main factors that may contribute to the lower incidence of AT in OHT compared with other thoracic surgeries are autonomic denervation and the use of steroids in OHT.
The common underlying mechanisms, prognosis and therapeutic strategies for each arrhythmia are described below.
Atrial Fibrillation
Early and late AF incidences and mechanisms (association with graft rejection), are shown in Table 2. In addition to small cohorts and variable follow-up durations, the definition of AF itself played an important role, as some studies counted brief episodes (33 % <6 minutes), while others included only sustained episodes.2 Moreover, the period used to link AF to acute rejection ranged from one week to one month.
These studies demonstrate that POAF (first month after surgery) is an uncommon finding (6 %), and is associated with AR in about one-third of patients. AF in this period was not associated with a poorer prognosis in OHT patients.
AF after the immediate post-operative period (after one month) was rare (4 %) but was associated with very poor outcomes. Indeed, almost no studies reported AF in stable OHT patients. AF in this context was mostly associated with AR in half of the cases and TCAD in almost one-quarter (and also diabetes).9 The remaining cases of AF in this context were linked to sepsis or multiple organ failure. Pavri et al.2 found that AF in OHT was associated with a three-fold increased risk of death (relative risk [RR] 3.15). Detailed analysis revealed that only late AF patients were affected and 100 % of them died. The reason for this is not clear, but it is likely that the more severe AR in this period, the cumulative effect of repeated AR episodes and TCAD may be determinant factors. In the absence of central autonomic factors, which increase dispersion of atrial refractoriness,10 and in the presence of pulmonary vein isolation, AF is more likely to be induced by a disease state of the myocardium (immune, ischaemic), or an abnormal neurohormonal milieu with an increased response to endogenous catecholamines11 and adenosine12 due to denervation. Acute changes caused by AR include oedema, lymphocytes infiltrate and myocardial necrosis; repeat episodes are associated with increased myocardial stiffness and fibrosis,13–15 which is likely to promote atrial heterogeneity and areas of slow conduction, facilitating AF.
Atrial Flutter
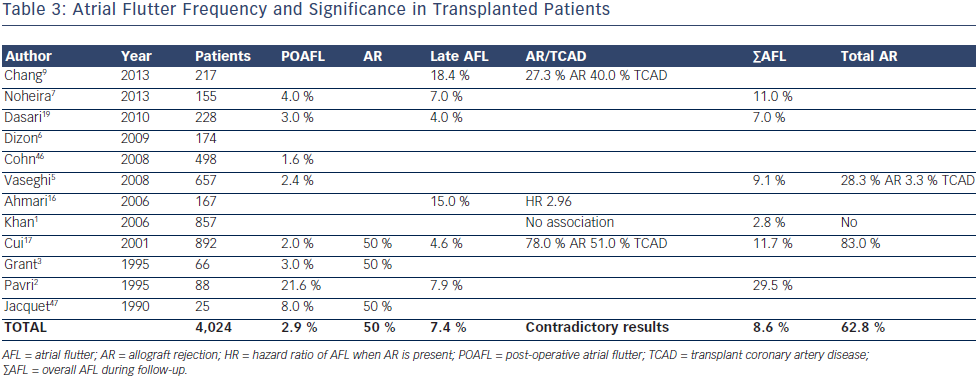
In the early post-operative period, AFL appeared to be even less common than AF (3 %) but the ratio AFL/AF (1/2) was much higher than after other thoracic surgeries (1/3–1/5)6 (see Table 3). Indeed, AR was more linked to POAFL than POAF (one every two versus one every three patients, respectively). A study by Ahmari et al.16 provided new insights, showing that acute rejection was associated with predominant increased pressure and size in the right rather than in the left atrium. The right ventricle is probably more affected by AR and, as with chronic lung diseases, is likely to increase the AFL/ AF ratio in such circumstances. Interestingly, no study has shown a correlation between left ventricular ejection fraction (LVEF) and SVT occurring in the first month during acute AR. Only myocardial biopsies provide a diagnosis of AR, with the sensitivity depending on the number and site.
On the other hand, AFL after the first month post-OHT is slightly more frequent (7 %) than AF and has not clearly been linked to AR. Vaseghi et al.5 and Chang et al.9 reported that 28 % of overall and late AFL, respectively, were associated with AR, but no comparison was done with sinus rhythm patients. Khan et al.1 did not find any association with rejection, but the Cui et al.17 study yielded a clear link when mild rejection (Grade 1–2 of the International Society for Heart and Lung Transplantation classification18) was also considered. In contrast to AF, no study has shown increased mortality in OHT patients experiencing AFL. In the Pavri et al.2 study a nonsignificant trend was weakened further by the fact that 78 % of those who died also had an episode of AF. Dasari et al.19 showed a significant increase in mortality associated with late arrhythmias only, but this analysis combined AF and AFL. Finally, Vaseghi et al.5 reported, in their stable (no AR) OHT cohort with SVT refractory to medical therapy, that 58 % were AFL. This was consistent with other studies20,21 where most stable OHT patients referred for ablation had cavotricuspid isthmus flutter and all were ablated successfully without any recurrence.
Other Supraventricular Tachycardias
The following arrhythmias have been described only in stable OHT patients referred for electrophysiological testing and ablation,5,20,21 thus allowing for a precise diagnosis; however, the possibility that AR or TCAD may be triggers in some patients cannot be excluded.
After isthmus-dependent AFL, the most common reentrant arrhythmias are:
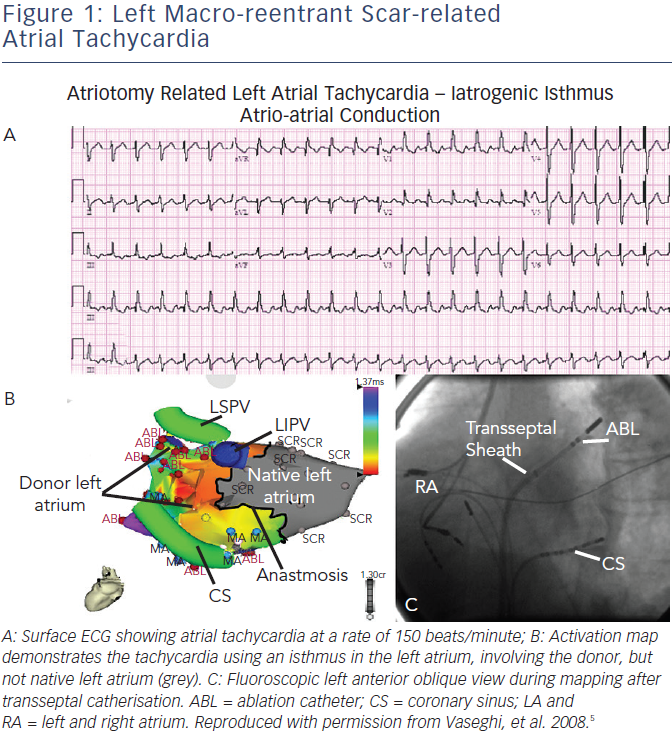
- Atrial macro-reentrant tachycardia (see Figure 1) – mostly occurring in the upper right atrium, around the native and donor suture line. The surgical scars at atrial suture lines can create areas of slower conduction, which were successfully targeted in all ablations. Such arrhythmias are typically the second group described, due to surgery (see Table 1), and they have promoted in part the development of bicaval anastomosis.
- Recipient-to-donor atrial conduction tachycardia also usually involved the right atrial anastomosis. In these cases, ablations were successfully performed at the site of the earliest donor atrial activation on the suture line and the recipient atria was not targeted. Further, recipient tachycardia or atrial rhythm with exit block to the donor atrium can challenge physicians with complex electrocardiograms (ECGs) showing dual atrial tachycardia,22 pseudo-atrioventricular (AV) block23 or a pseudo-atrial tachycardia with atrial waves of two different morphologies (one from the donor and one from the recipient).
- AV and AV nodal reentrant tachycardia with successful ablation of the accessory or slow atrioventricular pathways. We classified these (see Table 1) as arrhythmias that come with the transplanted heart, even if the donor never experienced any tachycardias; changes in autonomic tone affecting the substrate are likely to be the mechanism of tachycardias in the recipient patient.
Evaluation and Treatment
In the Post-operative Period (First Month)
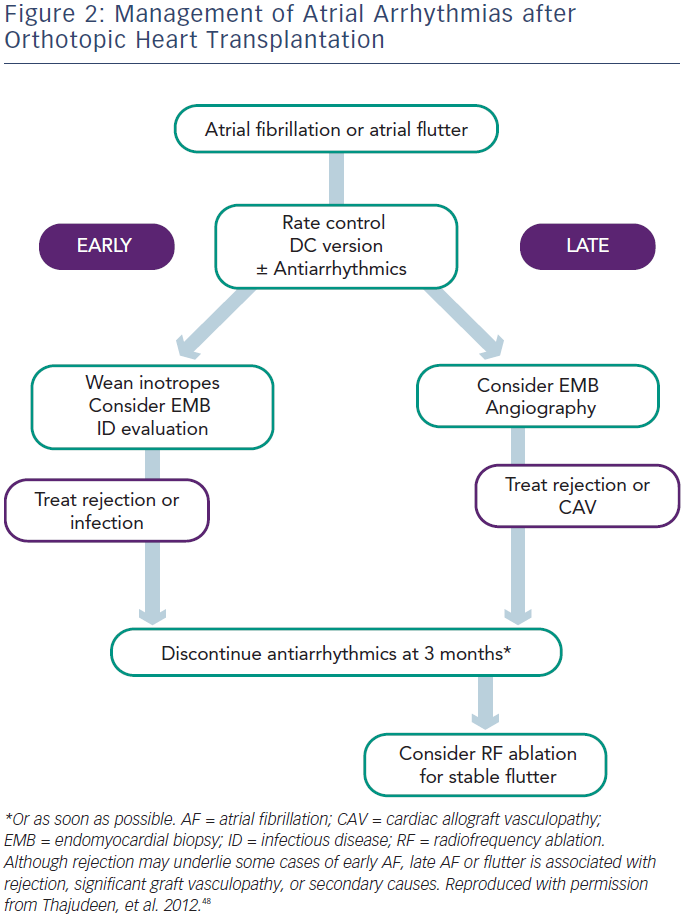
Symptomatic treatment with rate control (beta-blockers) and cardioversion as needed is preferred during this period. In all cases, optimisation of electrolyte status and weaning inotropes will benefit the patient. It is important to diagnose and treat AR and, in the case of persistent AF or AFL, repeating endomyocardial multisite biopsy may be helpful if the previous biopsy was negative. When anticoagulant therapy is needed, warfarin and dabigatran should be avoided because of the interaction with cyclosporin. Rivaroxaban and apixaban are probably the best choices in this setting. Use of antiarrhythmic drugs and calcium channel blockers is discouraged not only because of drug–drug interactions, but also because of the risk of severe bradycardia requiring pacing. Amiodarone should be avoided because of its prolonged effect, which may result in prolonged interaction needing monitoring of immunosuppressant levels, and prolonged sinus bradycardia requiring pacemaker implantation before patient discharge. When truly essential, class I antiarrhythmic drugs are most commonly used as TCAD is not expected in the early stage. It is important to note that in the Vaseghi et al.5 study none of the patients with POAF or POAFL had recurrence after cardioversion and drug discontinuation during follow-up, unless associated with repeat AR episodes or severe TCAD. Thus, without evidence of an increased risk of developing paroxysmal or persistent AF, antiarrhythmic drugs should be discontinued as soon as possible and anticoagulation discontinued one month later.
In the Late Period (After the First Month)
All patients with atrial arrhythmias without significant contraindications should receive anticoagulation therapy, irrespective of their CHADS2/ CHA2DS2-VASc score. In the study by Chang et al., in which patients received anticoagulation based on CHADS2 score, the group with atrial arrhythmias suffered more nonfatal cerebrovascular events compared with those in sinus rhythm (13.7 versus 3.6 %).9 Both AF and AFL patients should first be screened for AR and TCAD, with appropriate treatment. In the case of negative results, AF should be closely monitored with repeated biopsy and screened for sepsis. Antiarrhythmic drugs should be managed very carefully especially when patients have TCAD, and patients with stable paroxysmal or persistent AT should be considered for catheter ablation. In patients with persistent AT, anticoagulants should be discontinued one month after documented successful ablation, given the excellent follow-up results in these studies.5,20,21
Ventricular Tachycardia
Chang et al.9 analysed outcomes in patients experiencing ventricular tachycardia (VT; non-sustained and sustained) and have shown that among all arrhythmias this group had the poorest prognosis (89 % mortality at 83 months). The mean ejection fraction was normal and no patient died from a cardiovascular death; half of the deaths were associated with infection, one-third with AR and one-third with TCAD (there were some cases with both AR and TCAD). A case report24 described sustained bidirectional VT occurring during acute ischaemia in a patient with severe TCAD and ejection fraction (EF) of 40 %, requiring amiodarone, lidocaine and cardioversion. No VT was inducible with programmed stimulation and the patient underwent implantable cardioverter defibrillator (ICD) implantation but never had any appropriate therapy during follow-up. Thus, VT episodes should lead to careful evaluation of the underlying condition.
Bradyarrhythmias
Bradyarrhythmias occur in 8–23 % of patients after OHT depending on the case series. In most cases sinus node dysfunction is the main complication but AV block may also occur. The aetiology of sinus node dysfunction is variable and the transplantation technique itself can have a major influence. In the 1995 study of Grant et al.,3 66 patients survived more than 30 days, of which 35 underwent the biatrial anastomotic technique and 31 the bicaval technique. Three patients from the biatrial surgery group (8.6 %) received a permanent pacemaker while none from the bicaval technique group did. In a large United Network for Organ Sharing (UNOS) registry of almost 36,000 transplant patients reported in 2010, Cantillon et al.25 found that the bicaval technique was strongly protective against a need for a pacemaker (odds ratio, 0.33), and overall 10.9 % of the population required a pacemaker implant.
Transplanted heart autonomic changes with sympathetic and parasympathetic decentralisation/denervation can also lead to decreased sinus node automaticity, resulting in increased baseline and decreased maximum heart rate during exercise.26 Prolonged donor heart ischaemia time can predispose to conduction system injury in the post-operative period,23 while rejection can involve the cardiac conduction system and lead to bradyarrhythmias.27 Abnormalities of sinoatrial (SA) nodal artery may also influence sinus node dysfunction in patients who undergo OHT. One study performed routine coronary angiography in the first six weeks after the OHT and found that 30 % of SA nodal artery anomalies were in patients with implanted pacemakers and 6 % in the control group (without permanent pacemaker). In the same study, no correlation was found between graft ischaemia time and bradycardia.23 Sinus node dysfunction was described as sinus bradycardia in 17 %, sinus arrest in 27 % and junctional rhythm in 47 % of patients, while 63 % of the patients were asymptomatic and 73 % presented in the early post-operative period.
AV node dysfunction has similar multiple possible aetiologies in patients after OHT. It is most common in the late period after OHT. One study analysed the incidence of AV block among 1,047 patients finding first-degree AV block in 8.3 %, Mobitz I in 0.6 %, Mobitz II in 0.1 % and complete AV block in 1.8 %.28 Pre-operative use of amiodarone in the recipient patient may also result in post-transplant bradycardia. MacDonald at al. reported that these patients required longer periods of atrial pacing immediately post-transplant (mean seven versus three days), but that there was no effect of prior amiodarone therapy on inotropic function or clinical outcome.29
Evaluation and Treatment
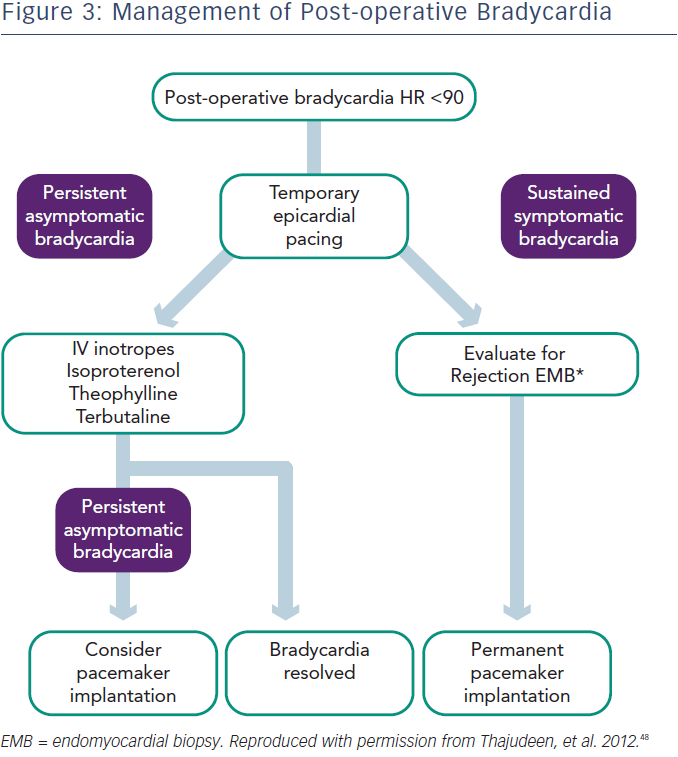
In the perioperative period bradycardia may be managed with temporary pacing.28 However, in up to 50 % of cases, sympathomimetics such as terbutaline or isoproterenol may be used to maintain the heart rate over 90 beats per minute, while waiting for recovery of sinus node function.30 In the case of sinus node dysfunction after heart transplantation, the current European Society of Cardiology (ESC) guidelines (2013) state that “a period of clinical observation from five days up to some weeks is indicated in order to assess if the rhythm disturbance resolves” (Class I recommendation, level of evidence C).31 Prompt permanent pacemaker implantation is indicated for symptomatic bradycardia and persistent second- or third-degree AV block. As previously noted, overall 10.9 % of patients in the UNOS database required pacemaker implantation.25 In cases of sustained bradycardia, consider endomyocardial biopsy to exclude the possibility of rejection.
In patients with late onset symptomatic bradycardia, rejection and TCAD should be excluded. In one study six of 18 patients underwent pacemaker implantation (three with sinus node dysfunction and three with AV block) and only two of them became dependent during follow-up. This study also found that the mechanism for late onset bradyarrhythmias is unclear, but 30 % of cases occurred in patients with TCAD, while acute rejection was rarely seen (5 %).9 TCAD was shown as a main reason for SCD, presenting with asystole and bradycardia.32 The optimal time for pacemaker implantation and prophylactic pacemaker implantation for bradycardia during the rejection period is not well defined. Permanent pacing is indicated for OHT patients with persistent, inappropriate or symptomatic bradycardia not expected to resolve, with a class I recommendation, level of evidence C, according to the American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) guidelines.33
Sudden Cardiac Death
Insights into SCD in OHT patients come from the Vaseghi et al. retrospective study34 of 628 patients, with 194 deaths, including 116 with a determined cause. In this cohort, one-third died of SCD, and interestingly, the terminal rhythm was asystole in 34 %, followed by pulseless electrical activity (PEA) in 20 % and ventricular fibrillation (VF) in only 10 % (unknown in 36 %). Almost two-thirds of SCDs were induced by acute ischaemia, and this subgroup yielded even more unexpected data since 50 % died from asystole, 44 % from PEA and only 6 % (one patient) from VF. In contrast, SCD in the general population is also associated with the same proportion of ischaemic heart disease (two-thirds), but VF is the most common mechanism occurring in up to two-thirds.35 Results in this OHT cohort could be prejudiced since it is difficult to rule out that VF degenerating to PEA or asystole occurred first, with PEA/asystole then recorded and counted as the initial rhythm. Nonetheless, among 11 patients implanted with ICDs for severe TCAD with depressed LVEF, out-of-hospital cardiac arrest, syncope or recurrent non-sustained ventricular tachycardia (NSVT), only one experienced an appropriate shock (which was possibly related to recurrent NSVT with an older defibrillator), while two patients received multiple inappropriate therapies (six shocks, five anti-tachycardia pacing).
Non-SCD patients in this cohort represented a very distinct profile as they died shortly after OHT (16 versus 38 months for SCD) and mostly from sepsis (43 versus 0 %), but almost never from ischaemia (4 %), and similarly from AR (13 versus 15 %). Again, the first documented rhythm was asystole (73 %).
As surgical techniques and immunosuppressive regimens have been refined, short-term mortality caused by sepsis has been replaced by increasing morbidity and mortality caused by TCAD. Prevention of SCD in this population has become a major concern in OHT care. The benefits of ICD implantation in OHT patients are limited and controversial. Indeed, patients who died from SCD had similar LVEF (48 %) to patients who died from non-SCD, which is not surprising considering that most patients died from asystole and not from VF. This is consistent with the cohort study of Leonelli et al.36 involving 89 patients, in which all five who died from SCD belonged to the group of patients with ECG evidence of progressive conduction system damage during follow-up (i.e. a new hemiblock or complete bundle branch block), of which two were directly related to bradycardia. All five patients had TCAD and the group experienced a mild deterioration of LVEF (62–55 %) during follow-up. The authors proposed that TCAD (or AR), which is known to affect the myocardium could also directly injure the conduction system and increase risk of SCD from bradycardia. On the other hand, Ptaszek et al.37 reported, in a cohort of 10 patients implanted 15 years after OHT, including five with severe decrease in LV function, that three of them had appropriate ICD therapy during follow-up. It is possible that reinnervation long after the surgery may have induced a higher susceptibility to ventricular arrhythmias with beneficial effect from ICD implant.
Improvements in the prevention and treatment of TCAD should be the cornerstone for decreasing mortality, and prospective studies are needed to enhance screening and choice of device. OHT patients with sinus node dysfunction should be implanted with a dual chamber pacemaker because of additional risk of asystole associated with TCAD. Furthermore, even TCAD patients who have a borderline indication for pacing such as paroxysmal, asymptomatic bradycardia or progressive conduction disease may benefit from pacemaker implantation, as procedural and infection risks probably do not counterbalance the risk of asystole and SCD. At least TCAD patients should undergo a careful assessment of the conduction system integrity by repeat ECG, Holter monitoring or electrophysiological studies leading to a multidisciplinary improved estimation of SCD risk. Finally, the use and benefit of beta-blocker therapy in TCAD patients should be carefully assessed in patients without pacemakers or ICDs because of asystole risk.
Interesting Pathophysiological Findings Associated with Arrhythmias in Orthotopic Heart Transplantation
OHT provides a unique model of the decentralised/denervated heart and reveals insights into the mechanism of arrhythmias. While one may assume that re-innervation occurs after OHT, Vaseghi et al.5 reported highly depressed heart rate variability parameters, even years after the graft surgery, which was consistent with previous studies.38,39 Specific findings are:
- The lower rate of peri-operative AF after OHT compared with other thoracic surgeries, with predominantly absence of AF in stable OHT patients, strongly supports both the autonomic and the pulmonary vein trigger hypothesis for genesis of AF. Moreover, AF episodes occurring long after transplantation are only associated with rejection or ischaemia, which alter the normal atrial substrate.17 As expected, during the acute post-operative state, only denervation is likely to decrease AF in OHT patients, as suggested by the higher rates after maze7 or double-lung transplantation6 both of which require pulmonary vein isolation.
- In the stable OHT cohort of Vaseghi et al.,5 among patients presenting with SVT referred for ablation, only 13 % were asymptomatic while 58 % were able to feel palpitations. Beyond a possible referral bias, this interesting finding supports the idea that palpitations may reflect chest wall rather than cardiac sensitivity.40
- In the same study, one of the 14 patients with AFL was associated with a tachycardia induced cardiomyopathy, which fully reversed after ablation. Hence the autonomic nervous system does not appear to be critical in the generation of tachycardia-mediated cardiomyopathy.
- In a second study by Vaseghi et al.34 VF as the mechanism of SCD was rare, even during acute ischaemia. Such findings highlight the critical role of the autonomic nervous system in the genesis and maintenance of malignant ventricular arrhythmias, particularly during ischaemia, which has been demonstrated for decades to induce a sympathetic reflex.41 Sympathetic hyperinnervation, which follows the denervation of scarred myocardium leads to a heterogeneous response to sympathetic stimulation causing an increased incidence of VT/VF.42,43 Furthermore, cervicothoracic sympathectomy, particularly bilateral, can have a significant antiarrhythmic effect.44,45 The decreased sympathetic reflex in the setting of ischaemia involving conduction system could also be a factor in asystole by decreasing the likelihood of an escape rhythm emerging.