Atrial fibrillation (AF) leads to a prothrombotic state1 and places patients at risk of thromboembolic disease. The most common and serious complication of thromboembolism is stroke, and AF is held responsible for 25 % of all strokes.2 Strokes in the context of AF are associated with a higher mortality, longer hospital stay and lower levels of independence at discharge.3 These factors combine to make antithrombotic therapy the most important management consideration for AF patients.
AF is a growing clinical problem owing to the ageing population, and the advances in treatment and improved survival of patients with cardiac disorders such as ischaemic heart disease and heart failure, which predispose to AF.4 It is estimated that, by 2050, up to 16 million people in the US will suffer from AF,5 which is already the commonest arrhythmia encountered in clinical practice.6 The healthcare costs are substantial and AF accounts for 1 % of the National Health Service expenditure in the UK.7
Most thromboembolic complications associated with AF can be prevented with anticoagulant therapy.8 For over fifty years, the only effective therapeutic option to prevent stroke in AF patients were vitamin K antagonists – i.e., warfarin. Although warfarin has been proved to be highly efficacious as thromboprophylaxis, its limitations have negatively affected physicians’ willingness to prescribe – and patients’ willingness to receive – this effective treatment.9 This variability in prescribing habits for thromboprophylaxis has seen large numbers of patients left unprotected against stroke or inadequately protected with antiplatelet therapy,10 despite evidence highlighting that aspirin is an inferior option and carries its own substantial bleeding risk.11 The last decade has therefore seen an intensive search for novel oral anticoagulant drugs that could overcome the limitations associated with warfarin and see more patients with AF properly protected against stroke.
In addition, there has been advancement in the risk stratification of patients with AF. Although AF increases the risk of stroke fivefold,12 this risk is not homogenous and depends on the presence or absence of specific stroke risk factors.13 These risk factors were incorporated into the simple CHADS2 score14 and used to artificially categorise patients as ‘low’, ’intermediate’ or ‘high’ risk. Traditionally, guidelines have recommended oral anticoagulation for high-risk patients, aspirin for low-risk patients, and either anticoagulation or aspirin for the intermediate-risk group. The CHADS2 score also classifies many patients as ‘intermediate-risk’,15 and there is evidence that warfarin is superior to aspirin for this group of patients.16 Similarly, the evidence suggests that aspirin is ineffective as stroke prevention for patients identified as ‘low-risk’ by CHADS2.17 Therefore the utilisation of the CHADS2 score in isolation would result in substantial numbers of patients at risk of stroke being undertreated.
These limitations of the CHADS2 score prompted efforts to establish a new schema that would be more inclusive of common stroke risk factors and would reliably identify ‘truly low-risk’ patients who do not require antithrombotic therapy, as well as reduce the number of patients at risk of stroke being denied oral anticoagulation.
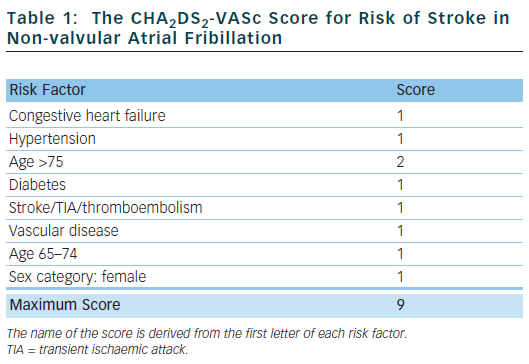
The CHA2DS2-VASc score18 has been proposed and, in various independent validation cohorts, has been shown to reliably identify ‘truly low-risk’ patients (annual stroke rate <1 % per year) and classes fewer patients as ‘intermediate-risk’.19 It also seems to be at least as good as – or possibly better than – the CHADS2 score at identifying ‘high-risk’ patients.19 This score has now been included in international guidelines.20
Table 1 outlines the risk factors that make up the CHA2DS2-VASc score. Congestive heart failure is defined as left ventricular ejection fraction ≤40 %. Hypertension is defined as blood pressure consistently above 140/90 mmHg or treated hypertension on medication. Vascular disease is defined as previous myocardial infarction, peripheral arterial disease or aortic plaque.
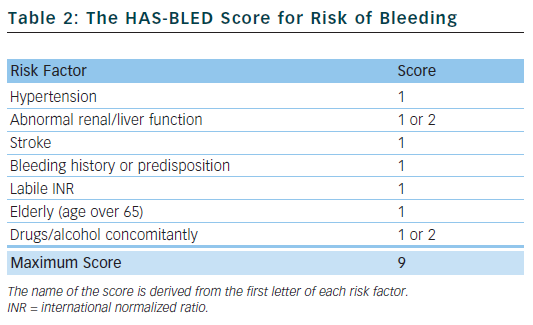
Bleeding is the most feared complication of antithrombotic therapy, and this can limit the prescription of oral anticoagulants.21 The HAS-BLED score22 is a simple tool to aid clinicians in undertaking a bleeding risk assessment and prompts them to consider the correctable risk factors for bleeding, such as labile international normalized ratios (INRs), uncontrolled hypertension and concomitant drugs. It can periodically be reassessed and has been validated in various large real-world cohorts,23 performing favourably compared with other bleeding risk scores.24 The HAS-BLED score has been incorporated into international guidelines.
Table 2 outlines the risk factors that make up the HAS-BLED score. Hypertension is defined as a systolic blood pressure >160 mmHg. One point is awarded for abnormal renal function and another point for abnormal liver function, and the same applies to drugs and/or alcohol. A score of 0–2 indicates a low risk of bleeding; a score ≥3 indicates a high risk of bleeding.
Many of the risk factors for stroke are also risk factors for bleeding and, at higher HAS-BLED scores, the net clinical benefit of warfarin is actually greater. This is because there is a greater and more dramatic reduction in the risk of ischaemic stroke and a comparatively smaller increase in the risk of bleeding when warfarin is given to patients with high HAS-BLED scores. Patients with CHA2DS2-VASc scores of 0 are truly low-risk and derive a negative net clinical benefit from warfarin.25
Warfarin
The efficacy of warfarin for preventing stroke and systemic embolism in AF patients was demonstrated in a string of randomised controlled trials (RCTs) over the preceding three decades, with a two-thirds risk reduction in ischaemic stroke compared with placebo. These data led to an increase in the use of warfarin and a consequent decrease in the number of strokes.26 As well as having a clear benefit over placebo, warfarin was also demonstrated to be superior to aspirin for stroke prevention in non-valvular AF.27 An RCT dedicated to evaluating stroke prevention in elderly patients with AF also showed the superiority of warfarin for the prevention of stroke, with no difference between warfarin and aspirin for major bleeding or intracranial haemorrhage.28
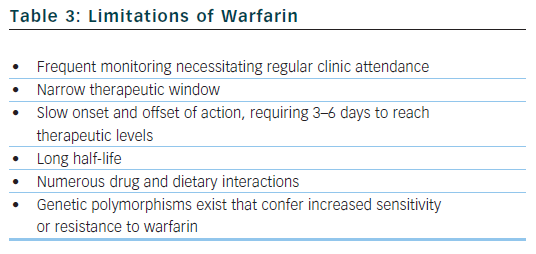
The benefit of warfarin is dependent on the amount of time that patients spend with their INRs in therapeutic range (TTR, time in therapeutic range).29 The optimal INR for patients with AF on warfarin for protection against thromboembolism has been established as 2.0–3.0, with an increased risk of thromboembolic and bleeding complications outside of this range. An increased TTR is associated with less thromboembolism, less bleeding, fewer myocardial infarctions and fewer deaths.30,31 Small improvements in TTR translate into significant benefits,32 with a low TTR potentially obliterating the benefit of anticoagulation. Self-monitoring can improve the quality of INR control33 and may bring the TTR closer to that achieved in clinical trials.34 Despite its unequivocal efficacy when properly used, warfarin has well documented limitations (see Table 3).35
Alternatives to warfarin must be proven to reliably perform at least as well as warfarin in RCTs, with an acceptable safety profile (ximelegatran was withdrawn due to hepatotoxicity36). New drugs should circumvent many of the limitations associated with warfarin that necessitate regular coagulation monitoring. They should therefore feature fixed-dose regimens, oral formulations, wide therapeutic windows, low propensity for food and drug interactions, and predictable pharmacokinetics and pharmacodynamics with little inter- and intra-patient variability.
Antiplatelet Therapy
In 2006, the ACTIVE-W (Atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events) compared dual antiplatelet therapy with aspirin and clopidogrel to warfarin for the prevention of thromboembolism in AF.37 The trial was stopped early due to the clear superiority of warfarin over dual antiplatelet therapy. Furthermore, the rates of bleeding in the two groups were very similar (2.4 % per year for clopidogrel and aspirin versus 2.2 % per year for warfarin). The evidence proves conclusively that antiplatelet therapy is an inferior option when compared with warfarin for stroke prevention. The comparable rates of bleeding also mean that, in patients deemed at too great a haemorrhagic risk, oral anticoagulation would not be a suitable treatment option.
Novel Oral Anticoagulants
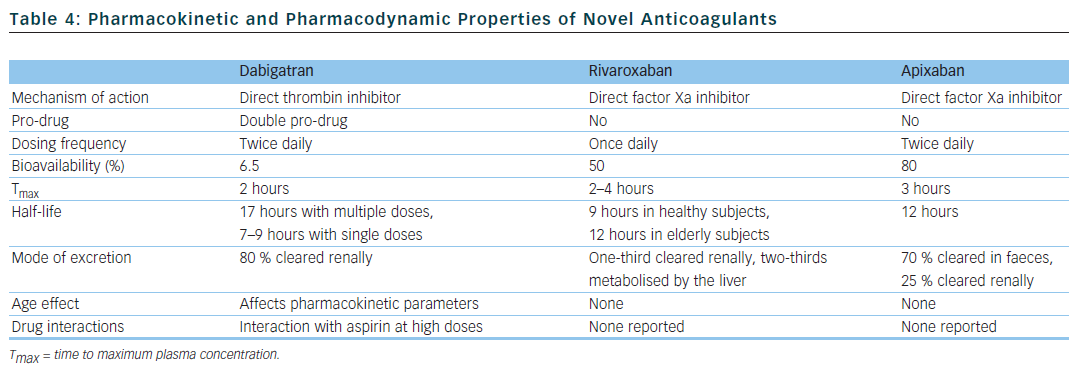
There have been concerted efforts to unearth new drugs that would fulfil the criteria for the ideal anticoagulant and could represent a viable alternative to warfarin. The three new drugs currently available fall into two classes: oral direct thrombin inhibitors (dabigatran) and oral factor Xa inhibitors (rivaroxaban and apixaban).38 Their pharmacokinetic and pharmacodynamic properties are summarised in Table 4.
Dabigatran
Dabigatran is a competitive, direct and reversible inhibitor of thrombin. The RE-LY (Randomized evaluation of long term anticoagulant therapy with dabigatran etexilate) trial was a large RCT comparing dabigatran with warfarin.39 This Phase III, blinded, non-inferiority trial studied 18,113 patients, either with non-valvular AF with a CHADS2 score of 1 or higher, or aged over 65 years with coronary artery disease. Patients were randomised to either dabigatran at a dosage of 110 or 150 mg twice daily or warfarin (INR of 2–3). The primary efficacy outcomes were stroke or systemic embolism. They occurred at a rate of 1.69 % per year in patients assigned to warfarin, versus 1.53 % per year in patients assigned to dabigatran 110 mg (relative risk [RR] 0.91, 95 % confidence interval [CI] 0.74–1.11, p<0.001 for non-inferiority, p=0.34 for superiority compared with warfarin) and 1.11 % in patients assigned to dabigatran 150 mg (RR 0.66, 95 % CI 0.53–0.82, p<0.001 for non-inferiority and superiority compared with warfarin). Thus low-dose dabigatran was non-inferior to warfarin and high-dose dabigatran was superior to warfarin for preventing stroke.
The primary safety outcome was major bleeding (defined as a reduction of 2 g/dl in haemoglobin, transfusion requiring at least two units of blood, or symptomatic bleeding in a critical organ). Major bleeding occurred less frequently with dabigatran 110 mg, and rates of major bleeding were similar with dabigatran 150 mg and warfarin: major bleeding occurred at a rate of 3.36 % per year in patients taking warfarin, 3.11 % per year in patients taking high-dose dabigatran (RR 0.93, 95 % CI 0.81–1.07, p=0.031 versus warfarin) and 2.71 % in patients taking low-dose dabigatran (RR 0.8, 95 % CI 0.69–0.93, p=0.003 versus warfarin). Both doses of dabigatran were associated with a significantly reduced risk of haemorrhagic stroke and intracranial haemorrhage when compared with warfarin.
Warfarin was the better tolerated therapy, with dyspepsia the primary contributor to discontinuation. Discontinuation rates were 21 % in the dabigatran 110 mg group, 21 % in the dabigatran 150 mg group and 17 % in the warfarin group at the end of the second year (p<0.001 for dabigatran versus warfarin). High-dose dabigatran was also associated with an increased risk of gastrointestinal haemorrhage.
Compared with warfarin, dabigatran 150 mg was found to induce a small increase in the number of myocardial infarction events that was not statistically significant.40 Warfarin is protective against myocardial infarction,41 and the small numerical increase in myocardial infarction events with dabigatran must be interpreted in the context of the overall net clinical benefit of dabigatran over warfarin and the lack of any increase in new angina hospitalisations or revascularisations.42
Rivaroxaban
Rivaroxaban is an oral, reversible, direct factor Xa inhibitor.43 Although its half-life is 7–12 hours, factor Xa is inhibited for up to 24 hours. This permits once-daily dosing, which is in contrast with the other novel oral anticoagulants. ROCKET-AF (An efficacy and safety study of rivaroxaban with warfarin for the prevention of stroke and non-central nervous system systemic embolism in patients with non-valvular atrial fibrillation) was a Phase III, randomised, double-blind, non-inferiority trial comparing rivaroxaban (20 mg once daily or 15 mg once daily in patients with moderate renal impairment) with warfarin (INR 2.5) in over 14,000 patients with non-valvular AF and a history of stroke, transient ischaemic attack (TIA) or non-central nervous system embolism or at least two independent risk factors for future stroke.44 Rivaroxaban was similar to warfarin for the primary endpoint of stroke or systemic embolism (event rate 1.71 % per 100 patient years with rivaroxaban versus 2.16 % with warfarin; hazard ratio [HR] 0.79, 95 % CI 0.66–0.96, p<0.001 for non-inferiority).
Bleeding was similar in the two groups (event rate 14.91 % per 100 patient years with rivaroxaban versus 14.52 % with warfarin; HR 1.03, 95 % CI 0.96–1.11, p=0.442). Rivaroxaban induced significantly less fatal bleeding and intracranial haemorrhage, but paradoxically significantly more patients on rivaroxaban required a blood transfusion or suffered a haemoglobin decrease of 2 g/dl or more.
The number of patients experiencing a serious adverse event was similar in the two groups (rivaroxaban 37.3 % versus warfarin 38.2 %), as was the documentation of an adverse event requiring discontinuation of the study drug (rivaroxaban 15.7 % versus warfarin 15.2 %). The trial’s population was generally high-risk, with 86 % of the total population possessing a CHADS2 score of 3 or higher. The quality of INR control, however, was less good, with an average TTR of 55 %.
Apixaban
Apixaban is an oral, selective and reversible direct factor Xa inhibitor.45 The ARISTOTLE (Apixaban for the prevention of stroke in subjects with atrial fibrillation) study was a randomised, Phase III, double-blind trial comparing apixaban 5 mg twice daily with warfarin titrated to an INR between 2 and 3 in over 18,000 patients.46 The primary outcome was stroke (either ischaemic or haemorrhagic) or systemic embolism, which occurred at a rate of 1.27 % per year in the apixaban group versus 1.60 % per year in the warfarin group (HR 0.79, 95 % CI 0.66–0.95, p<0.001 for non-inferiority, p=0.01 for superiority). This was primarily driven by a reduction in haemorrhagic stroke, which occurred at a rate of 0.24 % per year in the apixaban group versus 0.47 % per year in the warfarin group (HR 0.51, 95 % CI 0.35–0.75, p<0.001), as the rates of ischaemic stroke were similar (0.97 % per year in the apixaban group versus 1.05 % per year in the warfarin group [HR 0.92, 95 % CI 0.74–1.13, p=0.42]).
Apixaban demonstrated a benefit with regards to all-cause mortality compared with warfarin: rates of death from any cause were 3.52 % in the apixaban group versus 3.94 % in the warfarin group (HR 0.89, 95 % CI 0.80–0.99, p=0.047). Apixaban caused less major bleeding: 2.13 % per year, versus 3.09 % per year in the warfarin group (HR 0.69, 95 % CI 0.60–0.80, p<0.001). Drug discontinuation occurred less frequently: 25.3 % with apixaban versus 27.5 % with warfarin (p=0.001). Thus apixaban was superior to warfarin in preventing stroke or systemic embolism, caused less bleeding and resulted in lower mortality.
Limitations of Novel Oral Anticoagulants
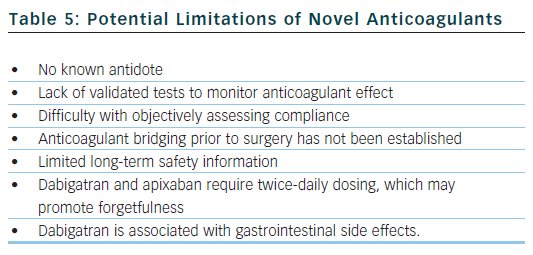
The arrival of these new agents represents a significant advancement in the treatment of AF, but clinicians must also be aware of the potential drawbacks and limitations of the new drugs (see Table 5). Dabigatran and apixaban require twice-daily dosing, which may lead to missed doses and place the patient in a pro-thrombotic state with increased risk of thromboembolism. Furthermore, anticoagulation monitoring is not required, so it is difficult to objectively evaluate a patient’s compliance with treatment.
Dabigatran has the propensity to cause dyspepsia and gastrointestinal haemorrhage (likely caused by its acidic core), which may limit its use in patients with gastrointestinal upset. Dabigatran and rivaroxaban must be used with caution in patients with moderate renal dysfunction, and dabigatran is contraindicated in patients with severe renal insufficiency. The 75 mg twice daily dose of dabigatran approved by the US Food and Drug Administration in renal impairment was never actually studied in the RE-LY trial.47 A subgroup analysis of the RE-LY trial found that the rates of stroke and thromboembolism were increased in patients with impaired renal function. Renal function should be checked periodically or if a decline may be suspected (commencement of nephrotoxic drugs, dehydration, intercurrent illness).
When managing a haemorrhage associated with the use of warfarin, or in other scenarios when prompt reversal of anticoagulation is necessary, there is an established antidote in the form of vitamin K. There are no known antidotes currently available in clinical practice for dabigatran, rivaroxaban or apixaban. Preliminary work is being done on a potential factor Xa inhibitor antidote,48 which works as a decoy type molecule. A Phase IV study is investigating various reversal strategies for dabigatran,49 and there is interest around an antibody against the fragment antigen-binding (FAB fragment) of dabigatran being used as a potential antidote: this may work in a similar manner to antibodies used against digoxin in cases of digoxin toxicity. It is hoped that antidotes will be available for use in the next three to five years.
The absence of an established antidote is important but must be kept in perspective: there are myriad antithrombotics used in cardiovascular medicine today – including aspirin, clopidogrel, bivalirudin and tirofiban – that do not have an absolute antidote available. The general management strategies of haemorrhage on a novel anticoagulant may include stopping the drug, local measures to cease bleeding, intravenous fluid and blood product support, maintaining a diuresis for renally excreted drugs and the use of haemostatic agents such as prothrombin complex where necessary.
Furthermore, there is no evidence base to guide management in certain specific challenging clinical scenarios, such as an intentional overdose of anticoagulants, switching between warfarin and new agents, major bleeding, and anticoagulant bridging in the peri-operative period. Clinicians can, however, draw on expert consensus on how to manage these situations with dabigatran,50,51 as well as on a position document from the Italian Federation of Thrombosis Centers.52
Conclusions
Clinicians now have an improved array of antithrombotic strategies to prevent stroke in AF patients, which should translate into improved outcomes for patients and a decline in the number of people suboptimally protected with antiplatelet therapy. New anticoagulants have already been incorporated into international guidelines, and physicians will therefore be tasked with choosing between a number of oral agents. While it is always difficult to infer comparisons between drugs that have not been tested against each other, there are some differences in the evidence, which clinicians may use to help determine the optimal antithrombotic strategy.
Rivaroxaban and apixaban have not been shown to cause dyspepsia, so may be the optimal option for patients unable to take dabigatran due to dyspepsia. When used in a low-dose twice-daily regimen, rivaroxaban also has a benefit for patients with a recent acute coronary syndrome (ACS),53 but the impact of the AF prophylaxis dose (20 mg once daily) plus dual antiplatelet therapy in the setting of an ACS has not been studied. Rivaroxaban is the only novel oral anticoagulant with once-daily dosing, which represents a small advantage over the other agents.
All three drugs also demonstrate positive bleeding profiles when compared with warfarin, especially intracranial haemorrhage and haemorrhagic stroke. Dabigatran is the only drug to significantly reduce the risk of ischaemic stroke.
Irrespective of the putative similarities and differences between the new antithrombotic agents, we are entering a new era for stroke prevention in AF patients which should result in improved outcomes, with more patients than ever receiving effective and safe thromboprophylaxis. Although there is limited evidence informing us on how to deal with specific scenarios, such as peri-operative bridging and anticoagulant overdose, all the safety data available are very reassuring. As our experience with these new drugs grows, we will better be able to deal with the challenges they present.